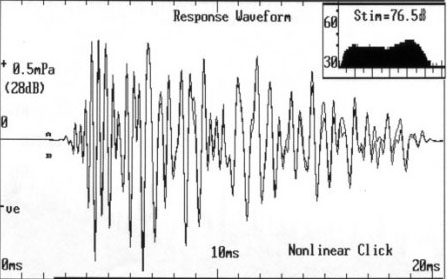
Otoacoustic emissions (OAEs) are acoustic signals generated by the active mechanics of cochlear outer hair cells. They can be measured with a small microphone probe inserted into the ear canal. Spontaneous OAEs can be detected in quiet but in most clinical applications OAEs are measured while the ear is stimulated with sound. Brief acoustic signals (e.g. clicks) can produce transient evoked otoacoustic emissions (TEOAEs) as illustrated in the panel on the left. Another type of emission is generated in the cochlea in response to two tone. In this case non-linear hair cell activity generates a distortion product, hence the name: distortion product otoacoustic emission (DPOAE).
OAEs are useful clinical and research tests because they can indicate the presence of functional outer hair cells. Subjects with normal hearing produce these emissions, however those with a cochlear hearing loss greater than 25 to 30 dB will have much reduced or absent OAEs. Because OAE testing is objective and easy to perform it has become an important part of newborn hearing screening programs. In infants, an absence of OAEs can be associated with hair cell dysfunction, however on occasion it can also be related to conductive loss such as the presence of fluid in the middle ear.
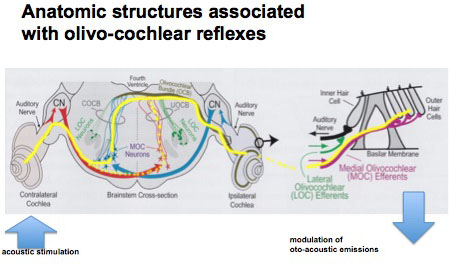
Cochlear outer hair cell mechanisms can be indirectly monitored by measurement of OAEs. A descending neural pathway originating in the brainstem (superior olive) can modulate the activity of hair cells. This is called the olivo-cochlear efferent system. This pathway can be activated by acoustic signals, and thus if OAEs are monitored in one ear, a sound presented to the opposite ear will reduce the OAE signal; this is often termed contralateral suppression of OAEs. The diagram on the left traces out the neural pathways for this system that can be considered as a “neural link between the ears”.
We have carried out a number of studies in animal models to explore some basic aspects of the olivo-cochlear efferent system. In addition to the effects of sound stimulation the modulation of hair cell activity can be influenced by level of anesthesia (HAREL, N., KAKIGI, A., HIRAKAWA, H., MOUNT, R.J. and HARRISON, R.V. Hearing Research (1997), and lesions to the olivo-cochlear nerve pathways (KAKIGI, A., HIRAKAWA, H., MOUNT, R.J. and HARRISON, R.V. Hearing Research (1997). We have also investigated in humans subjects the clinical utility of contralateral suppression (or modulation) of OAEs (e.g. JAMES AL, HARRISON RV, PIENKOWSKI M, DAJANI H, AND MOUNT RJ. International Audiology (2005).
Examples of our studies on “the neural link between the ears” are illustrated below. In the first example we have directly monitored DPOAE signals (in real time) while stimulating the opposite ear with sound. In the second study we have asked if contralateral suppression of DPOAEs is a feasible and useful clinical test to monitor age-related hearing deficits. At the bottom of this page a sample of our research papers are listed, with full PDFs available.
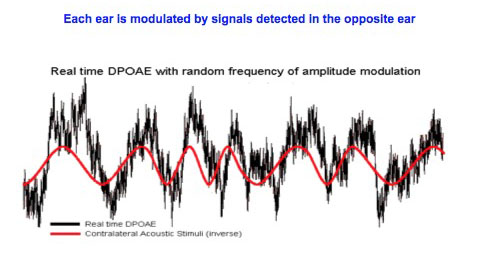
Amplitude modulation of DPOAEs by acoustic stimulation of the contralateral ear.
Otoacoustic emissions generated by outer hair cells (OHCs) are influenced by stimulation of the contralateral ear via a neural pathway involving the olivo-cochlear efferent system. This is often referred to as a contralateral ‘suppression reflex’, but we suggest that such a term is inappropriate since distortion product otoacoustic emissions (DPOAEs) can be both enhanced and suppressed, and there is continuous modulation with no threshold effects.
In the chinchilla, DPOAE amplitude is suppressed by an increase in contralateral stimulation, and enhanced by a decrease in sound. Thus, stimuli to one ear continuously modulate the OHC system (and therefore the biomechanical amplification) of the contralateral cochlea. In the right hand figure the DPOAE signal (black) is superimposed on an inverted acoustic signal waveform (red); note the clear inverse relationship between these opposite ear events.
(HARRISON RV, SHARMA A, BROWN T, JIWANI S and JAMES AL. Acta Otolaryngol (2008).)
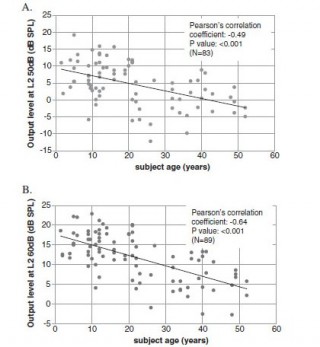
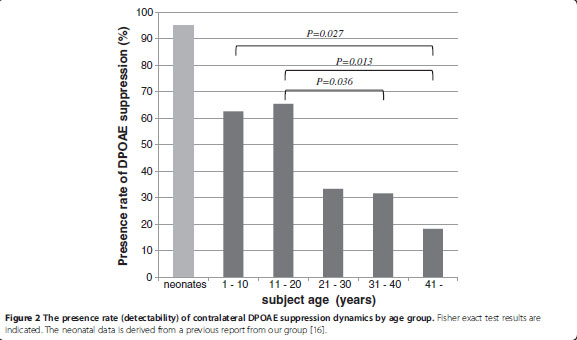
Age related changes to the dynamics of contralateral DPOAE suppression in human subjects.
This study was prompted by questions about the clinical utility of DPOAE suppression testing. Olivo-cochlear function as revealed by contralateral suppression of DPOAEs shows some important age-related changes. In addition to reduced emissions (outer haircell degeneration?) we find an increased latency that may reflect deterioration in auditory brainstem neural transmission. Regarding clinical utility, it is possible that the changes observed may reflect an aspect of age-related hearing loss that has not been previously considered. We conclude that in neonates and young children, contralateral suppression of DPOAEs can be reliably measured, and can potentially inform us about the integrity of OHCs, of cochlear afferent and efferent function and also auditory brainstem mechanisms.
KONOMI U., KANOTRA S., JAMES A.L. and HARRISON R.V. J. Otol. Head and Neck Surg. (2014).
References
Konomi U., Kanotra S., James A.L. and Harrison R.V. Age related changes to the dynamics of contralateral DPOAE suppression in human subjects. J. Otol. Head and Neck Surg. (2014). 43:15.
Papsin E., Harrison A.L., Carraro M. and Harrison R.V. Contralateral ear occlusion for improving the reliability of otoacoustic emission screening tests. Inter. J. Otolaryngology. (2014) 2014: 248187.
Wolter N.E., Harrison R.V. and James A.L. Separating the contributions of olivocochlear and middle ear muscle reflexes in modulation of distortion product otoacoustic emission levels. Audiology and Neurotology. (2014) 19: 41-48.
Harrison RV, Sharma A, Brown T, Jiwani S and James AL. Amplitude modulation of DPOAEs by acoustic stimulation of the contralateral ear. Acta Otolaryngol (2008) 128(4):404-7.)
James AL, Harrison RV, Pienkowski M, Dajani H, and Mount RJ. Dynamics of contralateral acoustic suppression of DPOAEs measured in real time in chinchilla and human subjects. International Audiology (2005)44:118-129)
Harel N., Kakigi A., Hirakawa H., Mount R.J. and Harrison R.V. The effects of anesthesia on otoacoustic emissions. Hearing Research (1997) 110, 25-33.
Kakigi A., Hirakawa H., Mount R.J. and Harrison R.V. The effect of crossed olivo-cochlear bundle (COCB) section on transient evoked otoacoustic emissions. Hearing Research (1997) 110, 34-38.