RareKids-CAN: The New Pediatric Rare Disease Clinical Trials and Treatment Network
RareKids-CAN: The New Pediatric Rare Disease Clinical Trials and Treatment Network
To ensure that every child, adolescent, and young adult in Canada affected by rare diseases has access to effective and innovative treatments.
Out of the 7,000 known rare diseases, only about 5% have specific treatments available. Patients and families often need to go to other countries and pay themselves for experimental treatments. RareKids-CAN wants to change this by supporting national and international clinical trials to make new discoveries and advance treatments for children, adolescents, young adults and their families here in Canada.
Funded in early 2024 by the Canadian Institutes of Health Research Institute of Genetics, as part of the Government of Canada’s National Strategy for Drugs for Rare Diseases investment, RareKids-CAN collaborates with patient/family partners, patient organizations, investigators, research networks, and industry partners to design, develop, set up, manage, and execute pediatric rare disease clinical trials in Canada.
To help develop and execute these cutting-edge clinical trials, the EnRICH Lab is involved and Dr. Offringa leads the RareKids-CAN Design and Methods Sub-Platform: https://www.rarekidscan.com/sub-platform-leads
Find Out More About RareKids-CAN
Winter 2026 Update:
In 2025, RareKids-CAN and its Methods Sub-Platform expanded their national infrastructure to accelerate paediatric rare disease clinical trials.
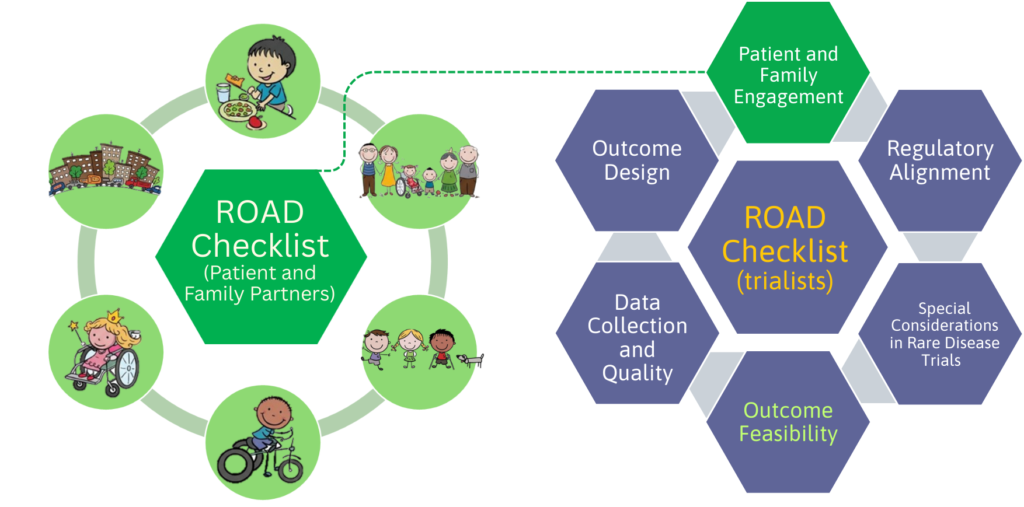
The Design and Methods Sub-Platform is leading the Rare Disease Clinical Trial Outcome Assessment and Design (ROAD) Project, a national and international initiative focused on improving how outcomes are designed, selected, measured, analysed, and reported in paediatric rare disease trials. The goal is to ensure that future trials generate evidence that is meaningful to patients and families, while also meeting modern scientific standards, international regulatory expectations, and industry needs.
What we are building
We are developing two ROAD Checklists to support best practices in outcome design for paediatric rare disease clinical trials:
- one for trialists, and
- one for patient and family partners,
to support meaningful partnership throughout trial design.
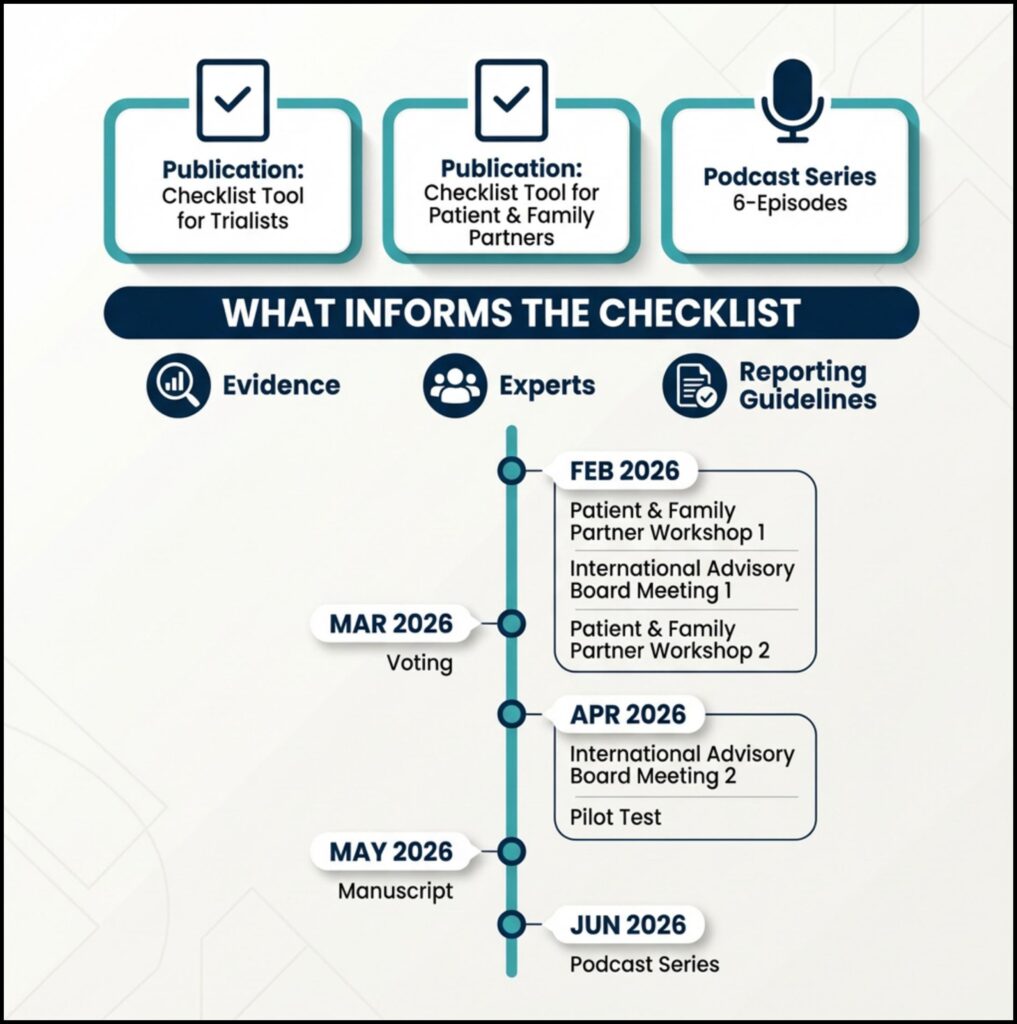
A draft checklist has been developed using:
- findings from a national and international needs survey of trialists;
- engaging with rare disease patient and family partners;
- consultation with methodological and clinical experts;
- review of relevant regulatory guidance (FDA and EMA);
- alignment with international trial reporting guidance, including SPIRIT-Outcomes, CONSORT-Outcomes, and SPIRIT-PRO.
What’s happening now (Winter–Spring 2026)
- February 2026:
- Patient and Family Partner Workshops to co-develop and refine outcome considerations.
- International Advisory Board (IAB) Meeting #1 to review the draft ROAD checklist and discuss integration of patient and family input.
- April 2026:
- International Advisory Board (IAB) Meeting #2 to validate revisions and move toward a final checklist.
These activities will be followed by a structured expert voting process, pilot testing of the checklist, and refinement of the accompanying manual
What’s next
More activities are planned for 2026, including:
- peer-reviewed publications describing the development of ROAD process;
- practical checklist tools for both trialists and patient & family partners;
- a podcast series focused on outcome design in paediatric rare disease trials.
Stay tuned for upcoming announcements and opportunities to get involved!
Updated February 6, 2026