What do we collect?
Tissues and clinical data on rare embryonal tumors
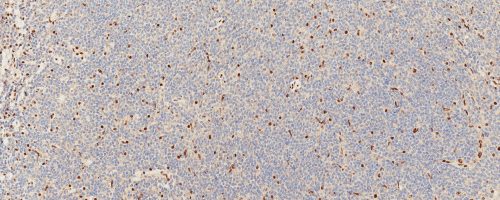
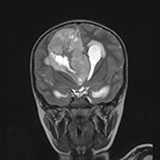
ATRTs (atypical teratoid rhabdoid tumors)
Also called rhabdoid tumors
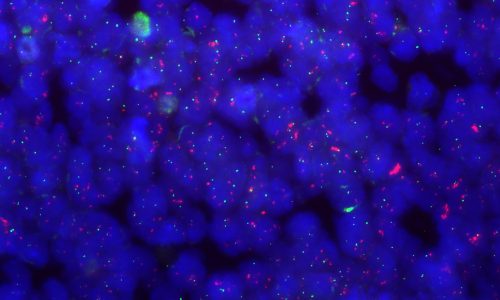
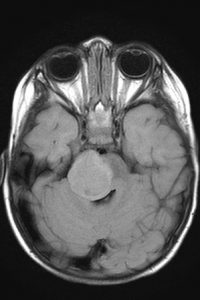
ETMRs (embryonal tumor with multiple rosettes)
Also called:
- C19MC-altered tumors
- Embryonal tumor with abundant neuropils and true rosettes (ETANTR)
- Medulloepithelioma
- Ependymoblastoma
Tumors formerly PNET, sPNET, or CNS-PNETs including:
- Embryonal tumor with FOXR2 activation
- Embryonal tumor with BCOR alteration
- Embryonal brain tumors NOS
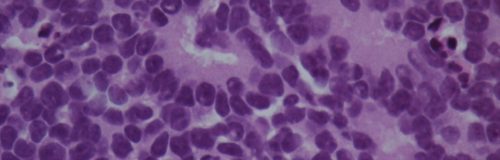
Pineal region tumors
- PPTID (pineal parenchymal tumor of intermediate differentiation)
- Pineoblastoma
We are interested in collecting some or all of the following:

1. Clinical data including:
- Demographics (age, sex, etc.), tumor location, staging, pathology reports, any molecular work-up data
- Imaging—MRI brain and spine imaging at diagnosis and relapse
- Treatment information and patient disease course
- Tumor or blood DNA and RNA
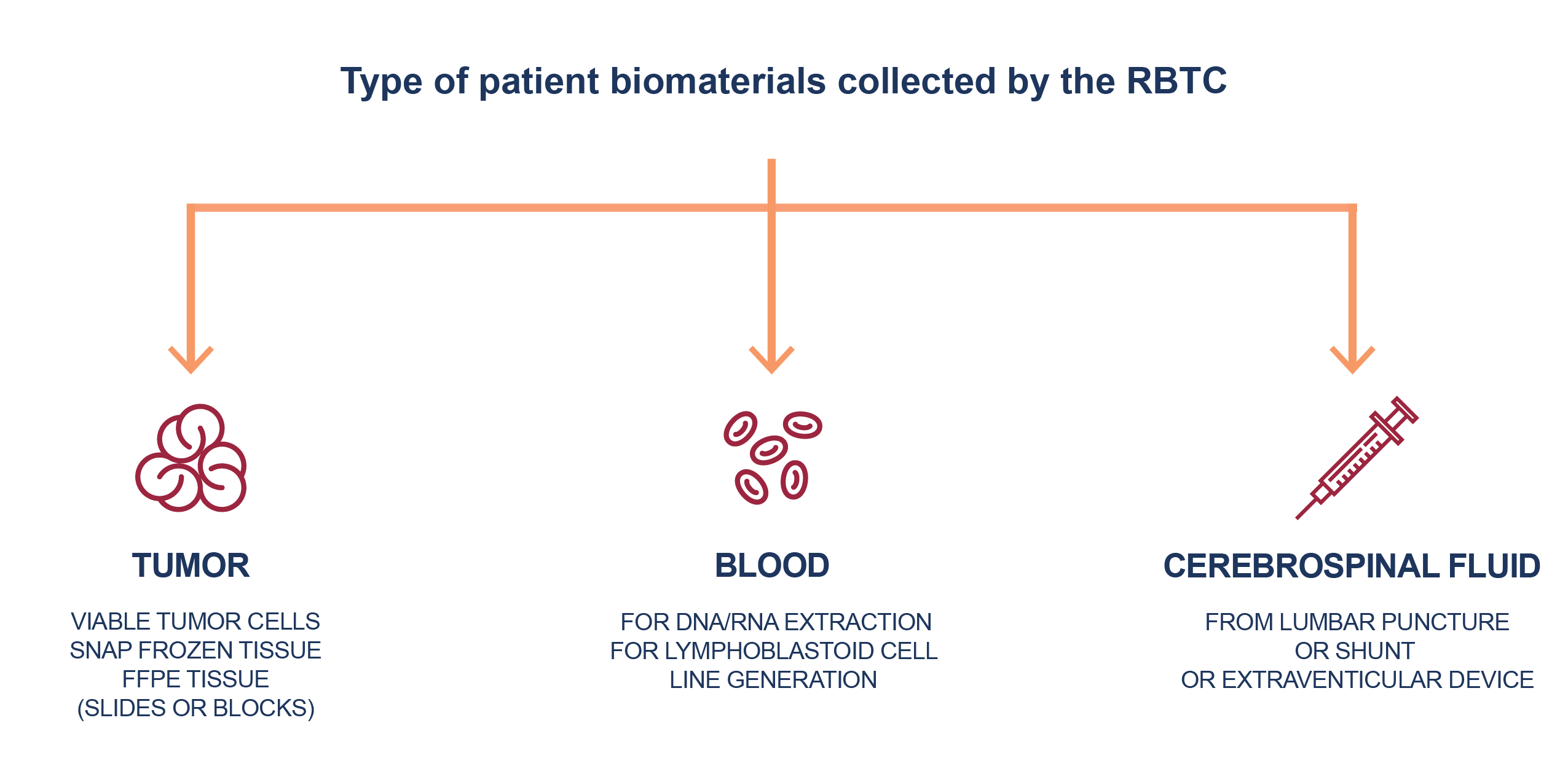
2. Biomaterials
- Tumor tissue in all available forms:
- Fresh and frozen tumor tissues – preferred
- Archived specimens preserved formalin and embedded in paraffin (FFPE); slides/blocks
- Patient tumor-derived cell lines or fresh viable tumor to establish cell lines or xenografts
- Blood
- Cerebrospinal fluid
- Tumor or blood DNA and RNA.
Biospecimen collection process
Please ensure tubes/samples are labeled in accordance to your hospital policies.
Please provide us with the following information separately:
- Unique identification number
- Gender and date of birth or age
- Date of collection
- Specimen source (CSF, lumbar, shunt or EVD)
- Time point of collection (diagnostic, intraoperative, or during therapy)
Please ship samples at the beginning of the week to:
Mei Lu/Huang Lab
Hospital for Sick Children
PGCRL 17,9410Q
72 Elm Street Toronto, ON M5G 1H3
Canada
Tel: 1-416 813 7654 ext.304362
Email: meilu@sickkids.ca
How do I enroll my patient and why?
If you have a patient with an expected or diagnosed rare brain tumor:
Option 1:
Contact us at rbt.consortium@sickkids.ca
Option 2:
Direct data entry—fill out our RedCap enrollment form!
- We will send you all necessary paperwork and instructions for collection of clinical data and preparation and shipping of biomaterials
- The RBTC covers shipping costs
- The RBTC will share any data concerning your patient, including molecular analyses once it is available
- You will become part of a global network and have opportunities to pursue research projects using RBTC data and resources
- All contributing collaborators share development of manuscripts/reports from the RBTC


