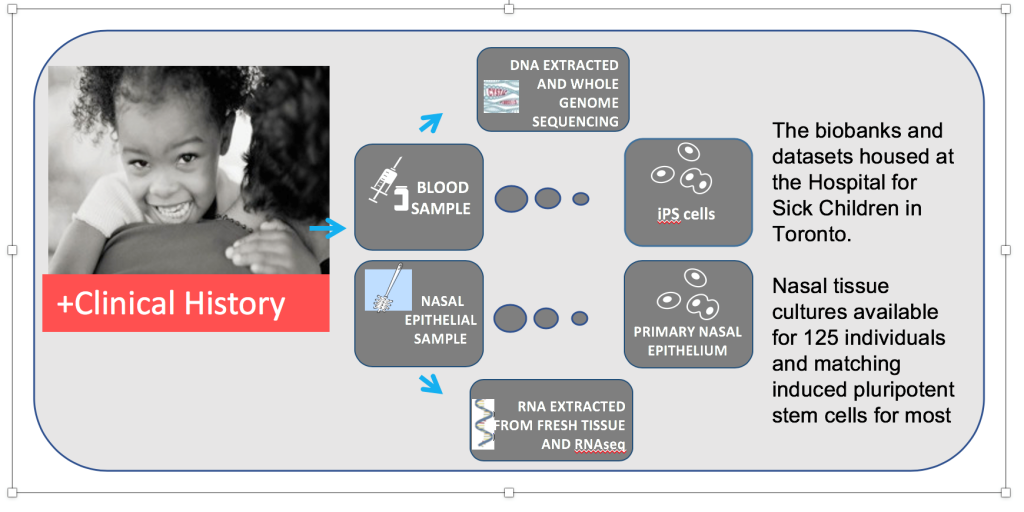
Figure S1: The CFIT resource. Sample collection includes nasal tissue for culture and RNAseq, blood collection for genome sequencing and generation of iPSCs, and clinical data collection for all 100 CF participants. Healthy control participants have nasal samples collected for culture and RNAseq, and blood banked for future iPSC generation.
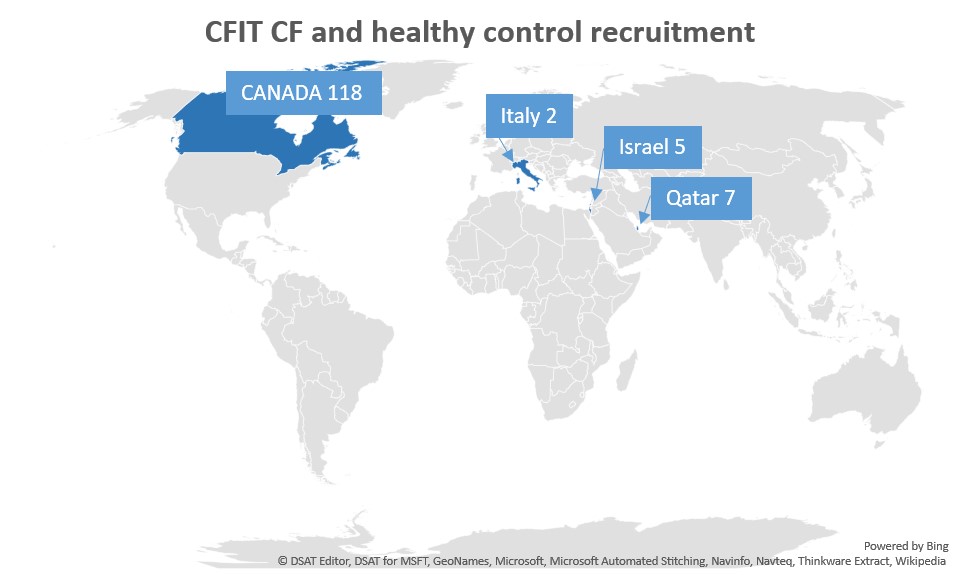
Figure S2. CFIT and healthy control recruitment worldwide. We have collected 118 participant samples in Canada and 14 samples from Italy, Israel and Qatar.
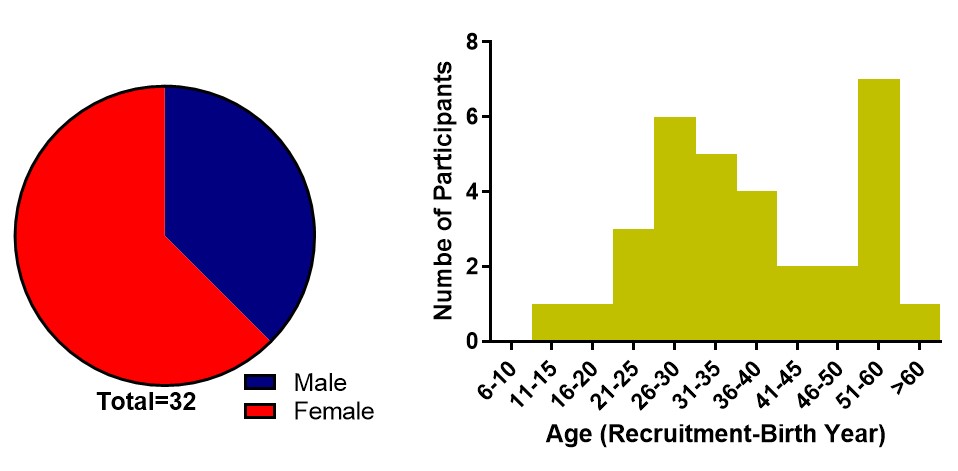
Figure S3. Characteristics of healthy control participants. Gender (left) and age (right) distribution of healthy control participants at time of initial sample collection.
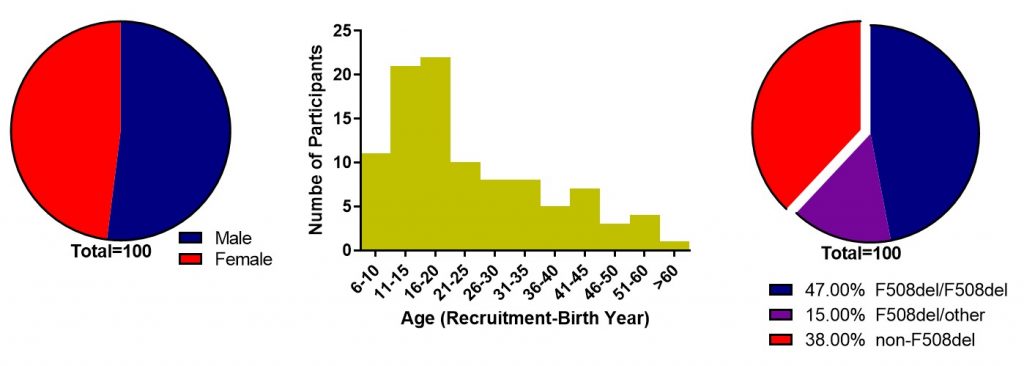
Figure S4. CFIT CF participant recruitment statistics. Gender at the time of first sample collection (left), age (centre), and CF genotype (right).
Table 1: Number of Participants with Select Homozygous CFTR Mutations
| Allele 1 | Allele 2 | Number of Participants |
|---|---|---|
| F508del | F508del | 47 |
| G85E | G85E | 3 |
| R334W | R334W | 2 |
| G551D | G551D | 1 |
| Y569D | Y569D | 1 |
| M1101K | M1101K | 5 |
| D1152H | D1152H | 1 |
| N1303K | N1303K | 2 |
| 621+1G>T | 621+1G>T | 4 |
| 3120+1G>A | 3120+1G>A | 1 |
| 3849+10KBC>T | 3849+10KBC>T | 1 |
| G542X | G542X | 3 |
| W1282X | W1282X | 8 |

