Research
Cardiomyopathy
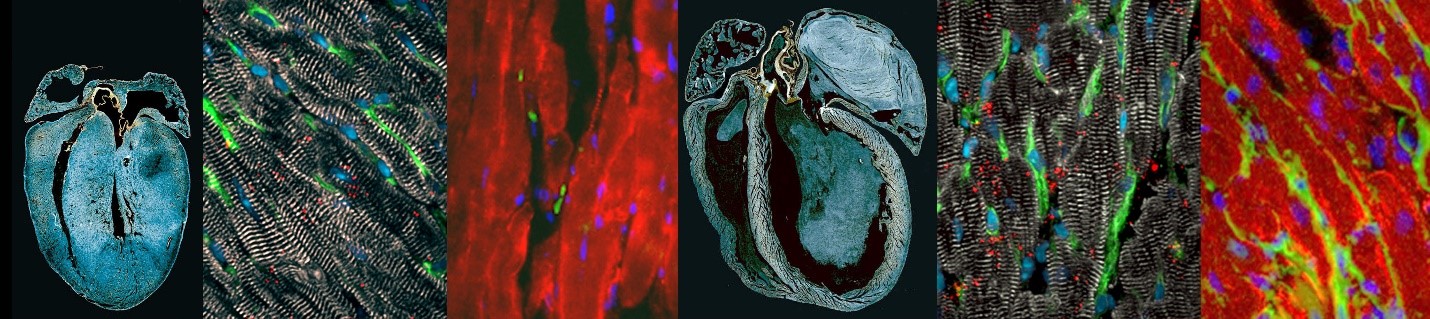
Diseases of the heart muscle, or cardiomyopathies, are a common cause of death. The most common of these is dilated cardiomyopathy (DCM). In DCM, the heart’s chambers become enlarged and the heart muscle stretches and thins, eventually reaching a point where the heart can no longer function. Right now, we do not know what causes DCM in about 70% of people who have it. These people are only diagnosed with DCM after their hearts have lost function. Our lab is investigating the events that occur before the heart loses function, and that drive initiation of DCM. We have generated mouse models carrying mutations in epigenetic regulators of gene expression, and that lose cardiac function just like people with DCM. We have discovered that, before it loses function, the heart changes the way it produces energy. By defining the early events that initiate deterioration of the heart towards DCM, we have identified potential markers for early diagnosis and experimental targets to prevent heart failure.
Publications
KDM8 epigenetically controls cardiac metabolism to prevent initiation of dilated cardiomyopathy. Ahmed A, Syed JN, Chi L, Wang Y, Perez-Romero C, Lee D, Kocaqi E, Caballero A, Yang J, Escalante-Cavarrubias Q, Ishimura A, Suzuki T, Aguilar-Arnal L, Gonzales GB, Kyoung-Han K, Delgado-Olguín P. Nature Cardiovascular Research. 2, 174-191 (2023). https://doi.org/10.1038/s44161-023-00214-0
Accompanying commentary in Nature Cardiovascular Research News & Views
Highlight in SickKids News & Stories
Highlight video by Lifespan News
Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Delgado-Olguín P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG, Tarakhovsky A, Bruneau BG*. Nat Genet. 2012 Jan 22;44(3):343-7. doi: 10.1038/ng.1068
Cardiovascular Development
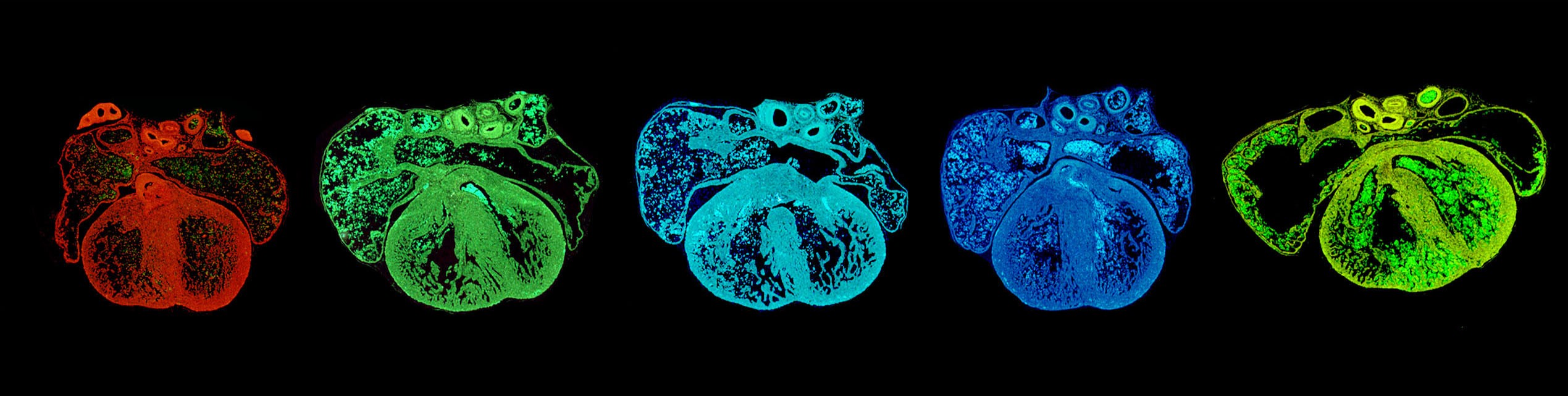
Cardiovascular abnormalities present at birth or manifesting in adulthood can originate during embryogenesis, but the specific cause of many of them remains elusive. Understanding the mechanisms controlling development of the cardiovascular system is essential for uncovering the origin of disease. Epigenetic regulators of gene expression have emerged as essential orchestrators of developmental pathways, but we have only started to understand their functions in the cardiovascular system. Our lab is investigating how epigenetic regulators, including histone modifiers, chromatin remodelers and organizers of the genome’s three-dimensional structure, control development of cardiac and vascular progenitor cells and their descendants.
Publications
Integrated small RNA, mRNA and protein omics reveal a miRNA network orchestrating metabolic maturation of the developing human heart. Aharon-Yariv A, Wang Y, Ahmed A, Delgado-Olguín P. BMC Genomics. 2023 Nov 23;24(1):709. doi: 10.1186/s12864-023-09801-8.
G9a inactivation in progenitor cells with Isl1-Cre with reduced recombinase activity models aspects of Dandy-Walker complex. Chi L, Zhong L, Lee D, Yu X, Caballero A, Nieman B, Delgado-Olguin P. Biol Open. 2023 Aug 15;12(8):bio059894. doi: 10.1242/bio.059894.
The transcriptional regulator CCCTC-binding factor limits oxidative stress in endothelial cells. Roy AR, Ahmed A, DiStefano PV, Chi L, Khyzha N, Galjart N, Wilson MD, Fish JE, Delgado-Olguín P*. J Biol Chem. 2018 Jun 1;293(22):8449-8461. doi: 10.1074/jbc.M117.814699
Ezh2-mediated repression of a transcriptional pathway upstream of Mmp9 maintains integrity of the developing vasculature. Delgado-Olguín P*, Dang LT, He D, Thomas S, Chi L, Sukonnik T, Khyzha N, Dobenecker MW, Fish JE, Bruneau BG. Development. 2014 Dec;141(23):4610-7. doi: 10.1242/dev.112607
Expression of NOL1/NOP2/sun domain (Nsun) RNA methyltransferase family genes in early mouse embryogenesis. Chi L, Delgado-Olguín P*. Gene Expr Patterns. 2013 Dec;13(8):319-27. doi: 10.1016/j.gep.2013.06.003
Embryonic Programming of Heart Disease
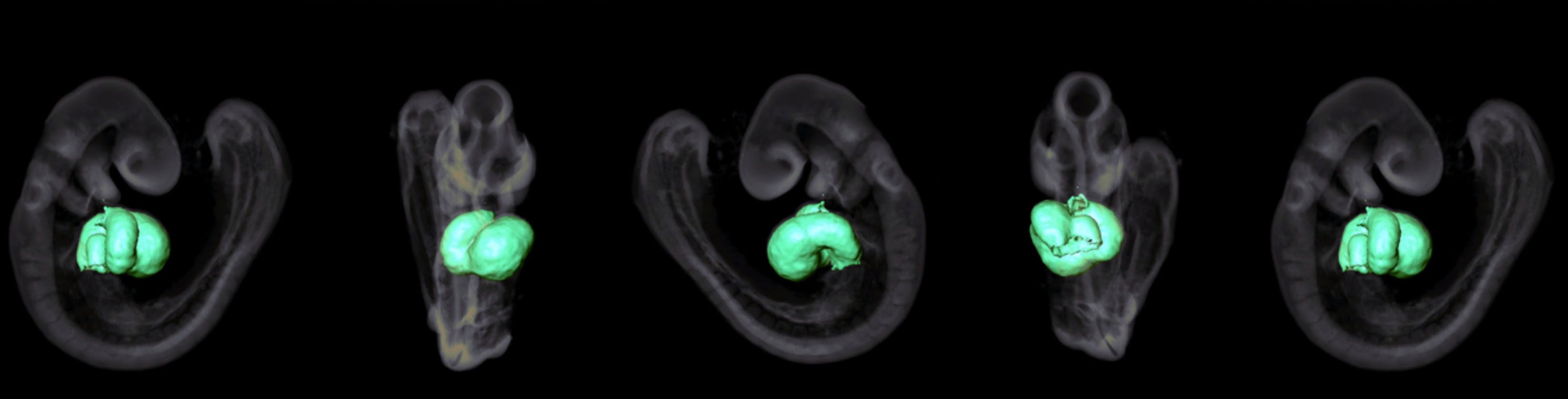
The risk of developing heart disease at an early age is higher in people if they were exposed to maternal obesity during fetal development. Because rates of obesity in women have increased over the years, and because of factors that stress the heart, children as young as 12 years old are at an increasing risk of early-onset heart disease. We do not yet understand how obesity during pregnancy makes the heart prone to disease. Once we do, we can use this knowledge to prevent heart disease in future generations. We are investigating the connection between maternal obesity and heart disease in the offspring. We are focused on uncovering the earliest effects of obesity during pregnancy on gene expression and the pathways that sensitize the heart to adult-onset disease.
Publications
Maternal obesity alters cardiac progenitor gene expression and programs adult-onset heart disease susceptibility. Ahmed A, Liang M, Chi L, Zhou YQ, Sled JG, Wilson MD Delgado-Olguin P*. Molecular Metabolism. 2021 Jan;43:101116. doi: 10.1016/j.molmet.2020.101116
Embryonic programming of heart disease in response to obesity during pregnancy. Ahmed A, Delgado-Olguin P*. Biochim Biophys Acta Mol Basis Dis. 2019 Feb 10;pii: S0925-4439(19)30040-7. doi: 10.1016/j.bbadis.2019.01.028
Placental Vasculature Maturation
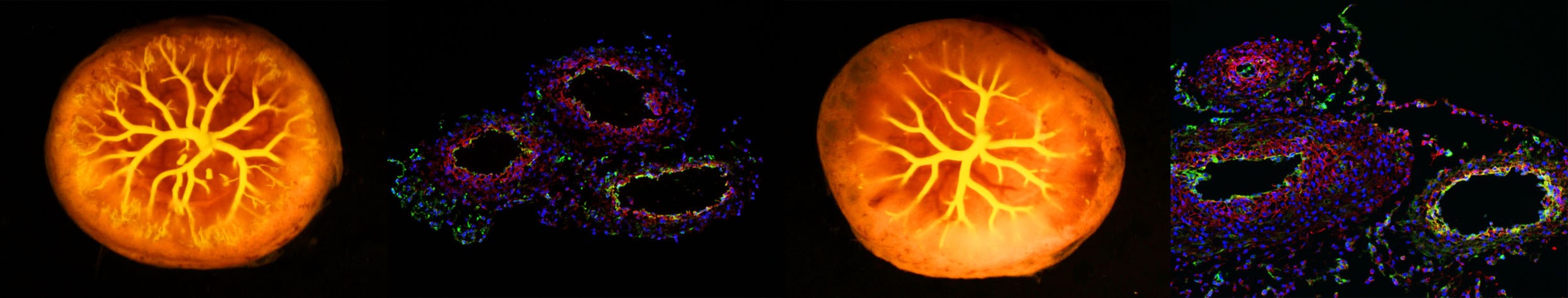
The placenta is a transitory organ essential for pregnancy and fetal development. To comply with nutritional demands of the growing fetus, the placenta transitions from a phase known as the developmental phase to a maturation phase. During maturation, the placental vasculature expands by elongation of the blood vessels. Abnormal maturation of the vasculature reduces placental function leading to restricted fetal growth and programming negative effects on the cardiovascular system’s health after birth. Our lab is investigating the cellular and molecular mechanisms that promote maturation of the placental vasculature. This knowledge will be leveraged towards strategies to prevent the negative effects of placental dysfunction on the postnatal cardiovascular system.
Publications
G9a controls placental vascular maturation by activating the Notch Pathway. Chi L, Ahmed A, Roy AR, Vuong S, Cahill LS, Caporiccio L, Sled JG, Caniggia I, Wilson MD, Delgado-Olguin P*. Development. 2017 Jun 1;144(11):1976-1987. doi: 10.1242/dev.148916.
Highlight in Development journal: Growing a labyrinth with G9a
Interview by The Node: The people behind the papers: Lijun Chi and Paul Delgado-Olguin
