Areas of Research – Neonatal Intestinal Disorders
Necrotizing enterocolitis (NEC)
Necrotizing enterocolitis (NEC) is our primary injury model. It is the most common gastrointestinal emergency in newborn infants, primarily affecting preterm and low birth weight neonates. The condition is characterized by ileal and/or colonic necrosis, ranging in severity from mild intestinal inflammation to intestinal perforation, multi-system organ failure and death. Despite advances in health research, the mortality rate from NEC remains above 30 per cent, and the pathogenesis of the disease is still not completely understood, demonstrating the need for continued study in this field.
Our research focuses on understanding the causes of NEC, while examining the effectiveness of various methods of prevention and treatment.
To evaluate a spectrum of intestinal injury models in neonatal rats, we compared different combinations of breastfeeding, maternal separation, hypoxia, lipopolysaccharide administration and hyperosmolar formula feeding. The combination of hyperosmolar feeding, lipopolysaccharide administration and hypoxia, induced the most representative NEC features of intestinal damage in the ileum and the colon (Zani et al., 2015). This is the combination of factors that we regularly use to create our experimental NEC model.
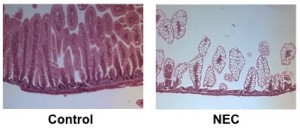
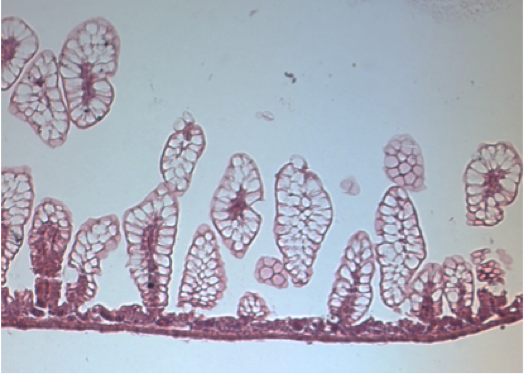
Hematoxylin and eosin staining of the ileum in control breastfed mice (left) and mice with experimental NEC (right)
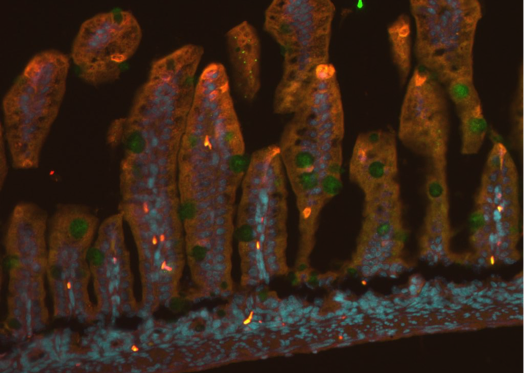
Hirschsprung’s Disease
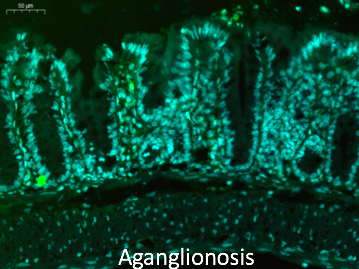
Hirschsprung’s disease is a common intestinal nervous system development dysfunction disease that is also known as aganglionosis. The incidence of Hirschsprung’s disease is approximately 1:5000. The aganglionic region produces a functional obstruction requiring treatment with surgical resection and removal of aganglionic bowel. However, over 50% of children have persistent problems post-surgery including severe constipation, fecal incontinence, and enterocolitis. The enteric nervous system (ENS) has a role in intestinal homeostasis, including motility, epithelial barrier function, mucosal immunity, epithelial transport, and regulation of the gut microbiome. Dysfunction of the ENS lead to the initiation or propagation of the inflammatory cycle of HAEC. These complications are more frequent in children with long-segment or total colonic Hirschsprung’s disease. Cell-based therapy offers the potential to introduce new neurons into the aganglionic colon as a novel therapy for this disease. Amniotic fluid stem cells (AFSC) – derived neuronal cell transplantation is also invaluable for Hirschsprung’s disease. Amniotic fluid is known to contain multiple cell types derived from the developing fetus. Cells within this heterogeneous population can give rise to diverse differentiated cells including those of adipose, muscle, bone and neuronal lineages. AFSCs are a potentially promising resource for cell therapy and tissue engineering.
Biliary Atresia
Biliary atresia (BA) is one of the most severe diseases of the liver and bile ducts in infants. Bile is a liquid that is produced in the liver and flows through the bile ducts to the intestines to assist with fat digestion. BA is characterized by bile duct damage, resulting in impaired flow of bile through the ducts from the liver to the gall bladder and intestines. Bile then accumulates in the liver, causing liver damage. The cause of BA is still largely unknown, and surgery is the only treatment currently used. The operation is called a Kasai procedure and consists of removal of the damaged bile duct and creation of a new duct. Despite successful Kasai procedures, approximately 50-80% of patients’ progress to liver failure and require liver transplantation, demonstrating the urgent need for a new therapeutic strategy. We have previously shown that AFSC have therapeutic effects in experimental models of other gastrointestinal diseases, such as necrotizing enterocolitis and ischemia/reperfusion injury. We hypothesize that AFSC have similar beneficial effects in BA. The primary objective of this project is to investigate whether these stem cells migrate to portions of the bile duct network located inside or outside of the liver, protect liver function and ultimately prevent the development of BA.
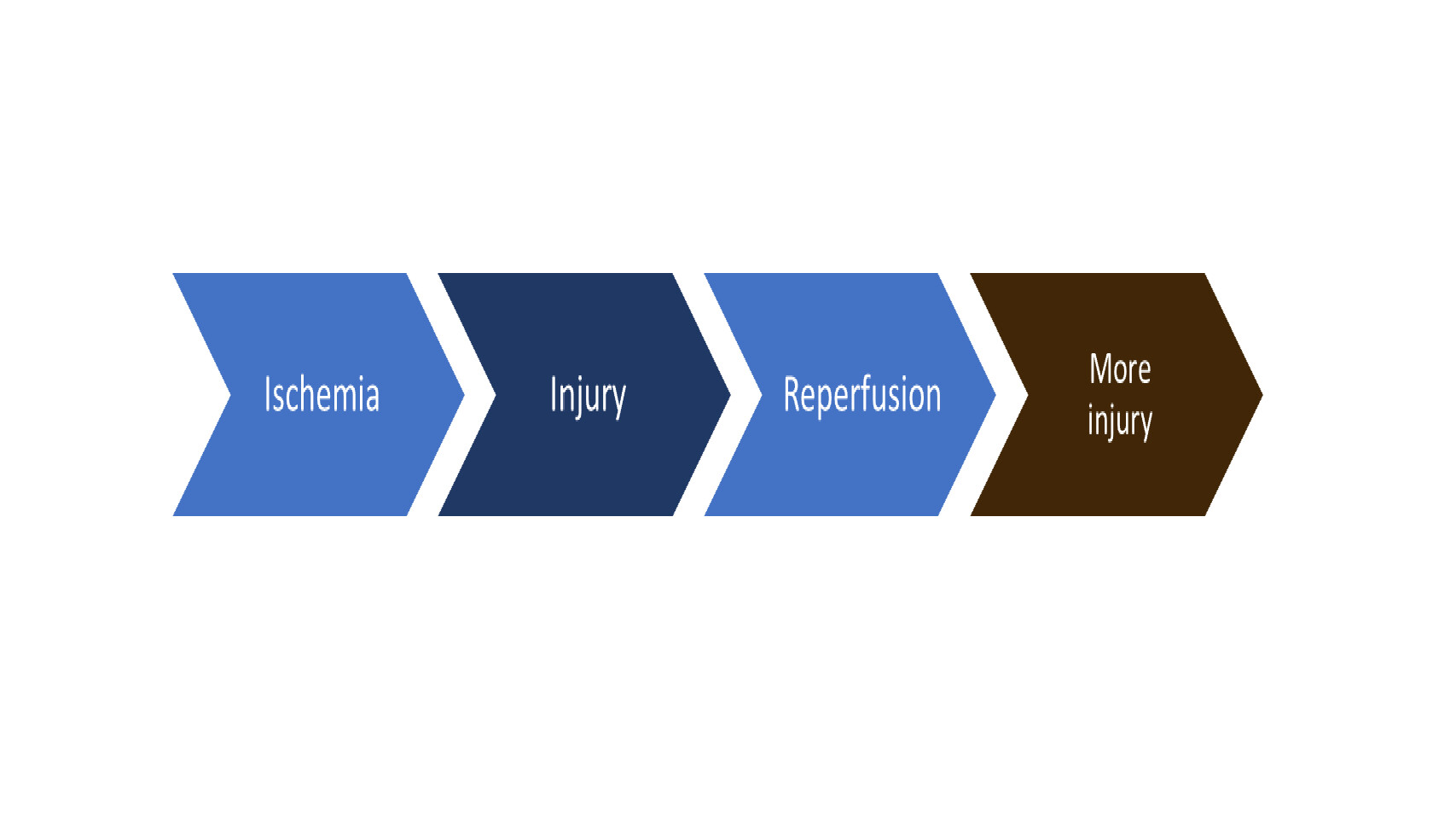
Ischemic Reperfusion
In the clinical setting, ischemic reperfusion (IR) can be present in infants and children with diseases such as midgut volvulus, intestinal intussusception, intestinal adhesive obstruction, and necrotizing enterocolitis. Some of these children develop intestinal necrosis following intestinal ischemia and need intestinal resection-anastomosis. It is important to investigate the effectiveness of stem cells, remote ischemic reperfusion (RIC) and organoid transplantation after IR and intestinal resection-anastomosis as these innovative therapeutic approaches can improve patient prognosis.
Maternal Separation (MS)
In neonatal intensive care units, very premature infants are regularly separated from their mothers, and cared for in incubators. Maternal separation is a well-established model of early life stress, and has been associated with changes in intestinal barrier and colonic epithelial dysfunction, thereby enhancing the risk of early intestinal illnesses.
We demonstrated that single and multiple separations of mice pups from their mothers resulted in significant changes in colonic morphology and bowel injury, characterized by crypt necrosis, compared to the non-separated mice. Additionally, we observed an increase in trans-cellular colonic permeability in the pups that underwent maternal separation (Li et al., 2014). More recently, our lab has demonstrated that maternal separation induces endoplasmic reticulum (ER) stress, and that treatment with ER stress inhibitors protects intestinal morphology and permeability (Li et al., 2016).
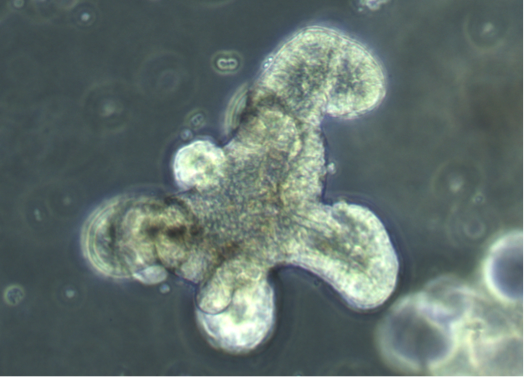
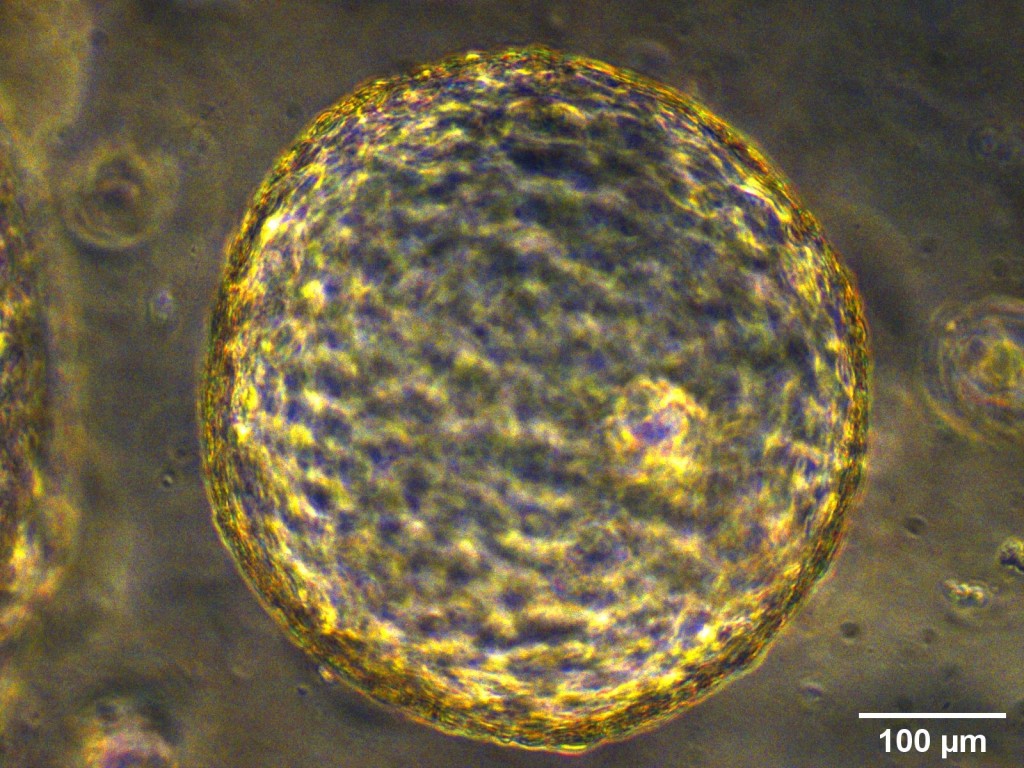
Intestinal Organoids
Organoids are self-organizing 3D structures grown from pluripotent stem cells (PSCs) or organ-specific adult stem cells (ASCs) in vitro, which resemble original tissue. ASCs-derived organoids can also be used in personalized medicine by allowing ex vivo testing of drug responses as well as development of transplantation methodologies for regenerative medicine and gene therapy. The pathogenesis of NEC remains unknown, but it is likely to be involved by multiple factors: gut immaturity, impaired gut barrier, circulatory instability, formula feeding, and disturbances of microbiome. Intestinal organoids derived from fetal intestine and human PSCs closely resemble immature human intestinal epithelium in ability of maturation, digestive functions and host-defense mechanisms. Thus, these organoids are a robust tool for modelling immature intestinal diseases such as NEC and the study of interactions that take place at the epithelial surface. Furthermore, intestinal organoids may have the potential to be used as source for transplantation due to their ability to engraft and rescue damaged intestinal epithelium after installation via intestinal lumen, and recent advances of tissue-engineered small intestine from intestinal organoids have provided a new hope of future autologous tissue transplantation for infants.
We are able to grow intestinal organoids from embryonic and postnatal mouse intestine and human biopsies. In addition, we also established liver ductal organoids which are mini-organ structures composed of cholangiocytes that harbor liver grogenitor cells and can recapitulate the functionality of in vivo bile ducts and are suitable for disease modeling.

Noble Gas Therapy: Argon Inhalation
Neonates with severe NEC are commonly intubated and mechanically ventilated creating an opportunity for the investigation of inhalation therapy in NEC. Argon is an emerging interest in the field of noble gas therapy and their organo-protective effects. There is much literature investigating xenon’s neuro-, cardio-, and nephron-protective properties, however, this noble gas is less abundant and extremely costly. Therefore, studies have gravitated towards argon’s similar protective properties being the more abundant and less costly option. In vitro and in vivo studies employing inhalatory argon mainly supports cardio- and neuroprotection.
In addition, various studies have proven argon inhalation to be safe in both adult and neonatal animal/human models. However, there is no current research investigation whether argon inhalation can treat against intestinal epithelial injuries, and those of neonatal disease, such as NEC. Importantly, we are of the first to test this treatment strategy in experimental NEC as well as neonatal mouse models. In this study, we aim to evaluate the protective effects of argon inhalation on acute the epithelial injury caused by NEC.
To address this aim we will develop the following objectives: 1) to ensure argon inhalation is safe in the neonatal mouse model; 2) to establish whether argon inhalation therapy reduces the acute injury caused by experimental NEC and how; and 3) to investigate whether argon can be neuroprotective of the brain.
Remote Ischemic Conditioning
Remote ischemic conditioning (RIC) is a therapeutic strategy that involves application of brief cycles of non-lethal ischemia and reperfusion to a remote organ, which helps distant organs withstand a subsequent ischemic injury. We recently demonstrated that in a neonatal mouse model of NEC, RIC rescues intestinal injury and inflammation and prolongs survival (Nat Commun. 2020;11(1):4950.) The mechanism of action of RIC involves improving the NEC-induced derangements in intestinal perfusion through enhanced vasodilation mediated by nitric oxide (NO) and hydrogen sulfide (H2S). RIC improves intestinal wall perfusion, as indicated by Doppler Ultrasound, and enhances intestinal microcirculation in the submucosa, as indicated by live in vivo recordings of submucosal blood flow obtained using two-photon laser scanning microscopy (TPLSM). RIC may have potential as a novel therapeutic option for NEC.
Stem Cells
Intestinal stem cells (ISC) initiate gut hemostasis and intestinal regeneration. We have demonstrated that Amniotic Fluid Stem cells (AFSC) rescue intestinal stem cell and stimulate epithelium regeneration in NEC through the Wnt pathway. This study highlighted the potential of stem cell therapy as a novel treatment for NEC. We have further explored this matter by using human stem cells derived compounds such as prostaglandin E2 (PGE2) to rescue intestinal damage.
Bioreactor
Bioreactor.
Brain Gut Axis
Brain.
Lipid Nanoparticles
Toll-like receptor 4 (TLR4) signaling is upregulated during NEC, causing an excessive inflammatory response and intestinal injury. Human milk-derived extracellular vesicles have been shown to prevent the development of experimental NEC. miRNA-148a, the most abundant miRNA found in milk extracellular vesicles, was shown to inhibit TLR4 signaling in other inflammatory conditions. To overcome the challenges of nucleic acid delivery, lipid nanoparticles (LNPs), made up of ionizable lipids, can be employed.