Ependymoma can be targeted by CAR T-cells
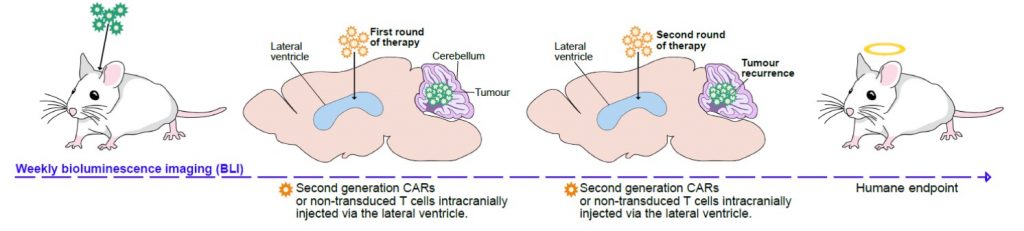
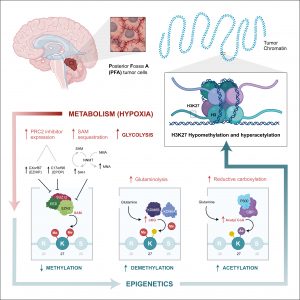
We have shown that PFA-ependymomas express very high levels of a molecule named HER2 on their surface. My team, in collaboration with the SU2C-USA Brain Immunotherapy Team, have evidence—recently accepted in Nature Medicine—of strong anti-tumor activity on human PFA-ependymoma, using immunotherapy. Cancer immunotherapy is basically the treatment of a person’s cancer using certain parts of a person’s own immune system to the fight disease. This can be done by either stimulating the patients own immune system to work harder to attack cancer cells. Or, in our case, by giving immune system components, such as man-made immune system cells. Immunotherapy has been used successfully to treat blood-borne cancers such as leukaemia and our experiments have shown that it can be adapted to work against solid tumours such as ependymoma. Single agents used to treat cancer often show an initial response followed by resistance. We believe that treatment with epigenetic agents with additional immunotherapy treatment will increase the killing of the cancer cells.
In this work we therefore propose to develop and evaluate the combination of epigenetic therapy with immunotherapy for PFA-ependymoma in order to avoid resistance and increase effectiveness. These experiments should lead to the development of a multicentre clinical trial combining these two therapies for infants with PFA-ependymoma. As there are currently no other options to treat children with PFA-ependymoma, such a trial would accrue quickly. Any survival advantage in the trial would rapidly allow this treatment strategy to become the standard of care for children with this devastating disease.