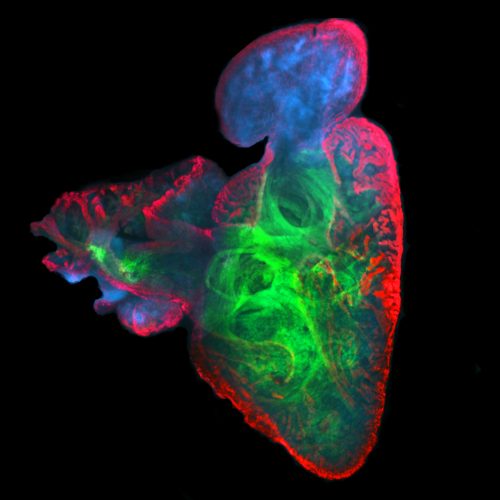
We use a number of approaches to carry out our research, including creation of numerous mutant and transgenic lines to examine gene function and image cells as they build the heart in real time. In collaboration with Dr. Michael Wilson at SickKids, we use sophisticated genomics approaches to discover genetic elements key to cardiac development and disease. By defining what is conserved between zebrafish and human, a gap that spans over 400 million years of evolution, we are starting to define the critical drivers of heart development and disease. Combined with the explosion of human genome sequences that are available, we aim to pinpoint the key genetic events that lead to cardiovascular disease, and that can be exploited for therapies. Further, the embryonic heart development program is re-deployed during adult zebrafish cardiac regeneration. We are therefore interested in studying the ability of key developmental gene regulatory networks to drive a regenerative response.
In the long term, this knowledge will be used to devise therapies based on activating regenerative pathways to heal the damaged heart, and using zebrafish embryos to screen for potential drugs to alleviate the burden of congenital heart defects and other diseases. Discovering how zebrafish cardiac progenitors are made and develop will therefore drive future efforts to repair what is at present irreparable heart disease. Given the common progenitors shared between the heart and craniofacial muscles, a further area of growing interest is in how pharyngeal mesoderm development is controlled and uncovering pathways that can lead to orofacial clefting and other forms of craniofacial malformations.
Trainees working on this project:
Nathan Stutt, Gwynn McLeod, Simon Monis (Wilson Lab)
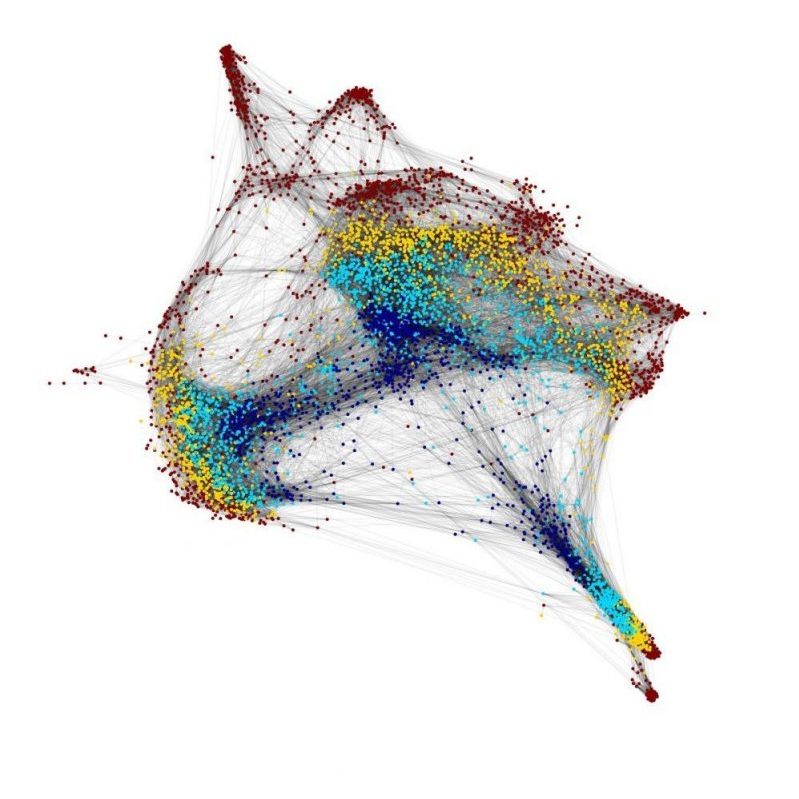
We have a major interest in the earliest events of heart development that regulate how cardiac progenitors, the building blocks of the heart, are first specified and diversified. Our early work with the grinch mutant has shown that function of the Apelin receptor GPCR (Aplnr) is essential for cardiac development, with aplnr/a/b mutants being heartless. This appears to be due to defects in the cardiac lineage at the earliest events of development, as gastrulation is progressing. To address this, we collaborate with the lab of Dr. Michael Wilson at SickKids to undertake single-cell RNAseq and ATACseq analysis of the initial stages of cardiac development in both wild type and heartless (aplnra/b and gata5/6 loss-of-function) models. We have found that regulation of gata5 expression is a key event in diversification of cardiopharyngeal lineages, a mechanism that we are actively studying. This work will lead to the key gene regulatory networks that govern the earliest events of cardiac lineage specification.
Trainees working on this project:
Esra Erkut, Casey Carlisle, Simon Monis (Wilson Lab), Laura McDonald
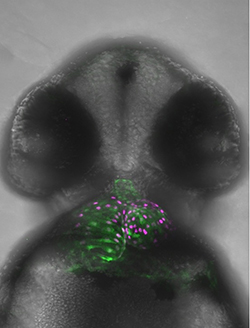
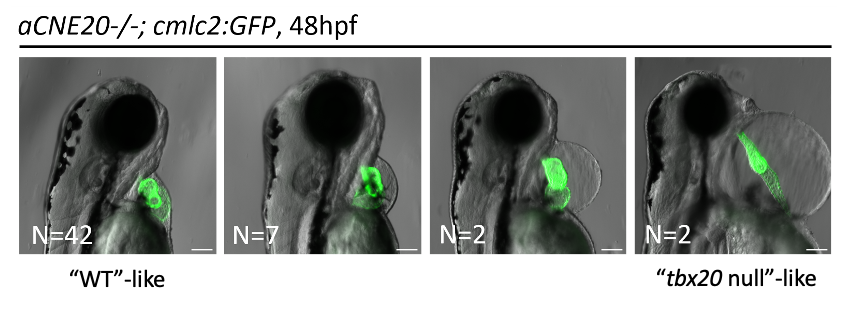
Subsequent to cardiac lineage commitment, cardiac progenitor cells must be diversified to form the various components of the heart. Our lab was amongst the first to demonstrate that zebrafish heart development occurs via two waves of addition of cells from the so-called first and second heart fields (FHF and SHF), as is true in mammals. Subsequently, these cells differentiate to form the structures of the heart. We are utilizing a comparative genomics approach to identify genes and gene regulatory elements (enhancers) conserved in cardiac expression/activity from zebrafish to human. These conserved elements are being examined for activity in transgenic and mutant models we are generating. We are in particular prioritizing enhancers with ultrarare variants in patients with congenital heart disease. Long-term goals include creating zebrafish models of congenital heart disease for functional and small molecule therapeutic screens, annotating non-coding variants associated with human disease, and identifying genes and enhancers that may play roles in cardiac regeneration.
Trainees working on this project:
Gwynn McLeod
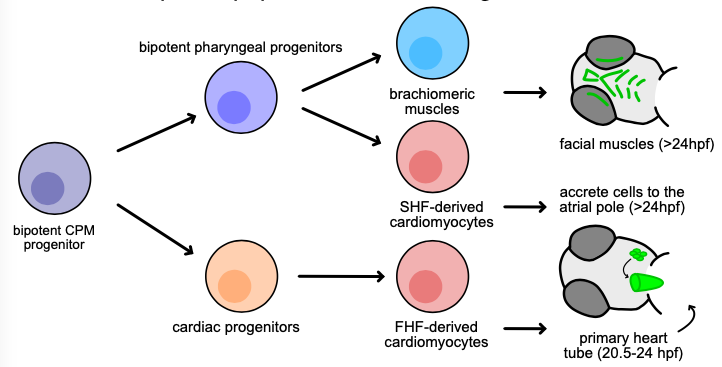
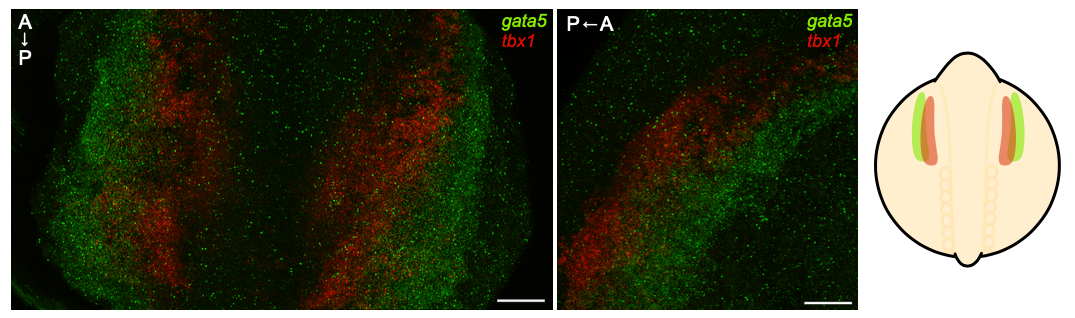
Our recent work has shown an important bifurcation in the development of an early cardiopharyngeal progenitor pool where gata5 expression is maintained in future cardiac cells while it is turned off in future pharyngeal mesoderm (where tbx1 is expressed). Given the known role of Tbx1 in pharyngeal and craniofacial development, we are further examining this key point in development by 1) defining regulatory elements that control gata5 and tbx1 expression; and 2) elucidating the gene regulatory network downstream of Tbx1 in pharyngeal progenitors. We are interesting is discovering how mutations in conserved gene regulatory elements and Tbx1 target genes can affect the severity of congenital heart disease and orofacial clefting including in 22q deletion syndrome (where TBX1 is affected).
- Early Cardiac Lineages
Trainees working on this project:
Nathan Stutt, Gwynn McLeod, Simon Monis (Wilson Lab)
We have a major interest in the earliest events of heart development that regulate how cardiac progenitors, the building blocks of the heart, are first specified and diversified. Our early work with the grinch mutant has shown that function of the Apelin receptor GPCR (Aplnr) is essential for cardiac development, with aplnr/a/b mutants being heartless. This appears to be due to defects in the cardiac lineage at the earliest events of development, as gastrulation is progressing. To address this, we collaborate with the lab of Dr. Michael Wilson at SickKids to undertake single-cell RNAseq and ATACseq analysis of the initial stages of cardiac development in both wild type and heartless (aplnra/b and gata5/6 loss-of-function) models. We have found that regulation of gata5 expression is a key event in diversification of cardiopharyngeal lineages, a mechanism that we are actively studying. This work will lead to the key gene regulatory networks that govern the earliest events of cardiac lineage specification.
- Conserved Enhancers in Heart Development and Disease
Trainees working on this project:
Esra Erkut, Casey Carlisle, Simon Monis (Wilson Lab), Laura McDonald
Subsequent to cardiac lineage commitment, cardiac progenitor cells must be diversified to form the various components of the heart. Our lab was amongst the first to demonstrate that zebrafish heart development occurs via two waves of addition of cells from the so-called first and second heart fields (FHF and SHF), as is true in mammals. Subsequently, these cells differentiate to form the structures of the heart. We are utilizing a comparative genomics approach to identify genes and gene regulatory elements (enhancers) conserved in cardiac expression/activity from zebrafish to human. These conserved elements are being examined for activity in transgenic and mutant models we are generating. We are in particular prioritizing enhancers with ultrarare variants in patients with congenital heart disease. Long-term goals include creating zebrafish models of congenital heart disease for functional and small molecule therapeutic screens, annotating non-coding variants associated with human disease, and identifying genes and enhancers that may play roles in cardiac regeneration.
- Cardiopharyngeal Development
Trainees working on this project:
Gwynn McLeod
Our recent work has shown an important bifurcation in the development of an early cardiopharyngeal progenitor pool where gata5 expression is maintained in future cardiac cells while it is turned off in future pharyngeal mesoderm (where tbx1 is expressed). Given the known role of Tbx1 in pharyngeal and craniofacial development, we are further examining this key point in development by 1) defining regulatory elements that control gata5 and tbx1 expression; and 2) elucidating the gene regulatory network downstream of Tbx1 in pharyngeal progenitors. We are interesting is discovering how mutations in conserved gene regulatory elements and Tbx1 target genes can affect the severity of congenital heart disease and orofacial clefting including in 22q deletion syndrome (where TBX1 is affected).