Mystery of Neurodegenerative Diseases
Neurodegenerative diseases are increasingly recognized as one of the leading causes of disability and death worldwide; however, no cure is available for these diseases. Insufficient understanding of the mechanisms underlying neurodegenerative diseases hinders development of effective treatment strategies. The key questions that remain enigmatic in the neurodegenerative research field are (1) how do neurons die?, (2) why certain neurons are selectively affected? and (3) does inflammatory response protect neurons from dying or accelerate the dying process? Elucidation of the neurodegenerative process, discovery of the vulnerable neuronal cell types and identification of the role of inflammation in the nervous system would facilitate development of powerful and effective strategies for therapeutic intervention.
Focus on ALS and the disease-causing gene MATR3
Our lab explores amyotrophic lateral sclerosis (ALS) to answer these questions. Many ALS genes have been discovered and continue being discovered (Zhao et al. Mol Cells 2018). Since our lab opened, we embarked on studying a novel ALS gene called Matrin 3 (MATR3), which encodes a nuclear matrix protein that is involved in RNA splicing. To understand its physiological role and the role of its pathogenic mutations, our lab established MATR3 mutant models using the two complementary model systems, fruit flies and mice.
Why use fruit flies?
The strength of fruit flies lies in the power of genetic screens and the availability of large genetic toolkit, enabling identification of conserved genetic pathways that are affected by the disease-causing proteins. Our lab generated fly models that express either the wildtype or the mutants of human MATR3 in the motor neurons and muscle. We found that the mutants exhibit more severe phenotypes than the wild-type MATR3, suggesting that the mutant flies will be useful for studying the mechanism of MATR3 toxicity. Through genetic screening, we discovered that axonal transport proteins are modifiers of mutant MATR3 toxicity, suggesting that axonal transport defects are involved in disease pathogenesis (Zhao et al. FEBS Letters 2020).
Mighty mouse – MATR3 S85C knock-in mouse model of ALS
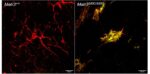
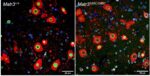
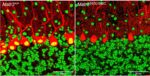
Mouse models have been a powerful tool for studying the molecular mechanism of neurodegenerative diseases as they can recapitulate many aspects of the diseases. Our lab created a new ALS mouse model, which recapitulates early stage features of human ALS including motor deficits, motor neuron defects and neuroinflammation (Kao et al. Nature Communications 2020). This mutant mouse line (MATR3 S85C knock-in mice) harbors a single ALS-linked missense mutation S85C in mouse Matr3 locus, closely mimicking the human genotype. In addition to recapitulating early aspects of ALS including motor neuron (neuromuscular junction) defects, MATR3 S85C knock-in mice exhibit severe loss of Purkinje cells. We also found a dramatic nuclear loss of MATR3 S85C in the neurons that undergo degeneration such as Purkinje cells and motor neurons, but not in neighboring cells. These results implicate that loss of MATR3 function contributes to neuronal defects and that the mutation impacts selective neuronal groups.
Our research goals
Together with these clinically relevant disease models, we will employ genetics, molecular and cell biology, RNA profiling and high-throughput genetic screens to
- identify the early disease events in the neurodegenerative process,
- elucidate the mechanism underlying selective neuronal vulnerability and
- determine the role of microglia in ALS pathogenesis during early-stage ALS.
Addressing these questions will advance our understanding of the disease mechanism and enable development of early intervention strategies for ALS.
In addition to disease research, we conduct basic research that addresses the fundamental questions by which an RNA binding protein controls gene expression or alternative splicing and thereby regulate neuronal development and neuronal health. Basic science research is essential for understanding the biological mechanisms underlying diseases and would accelerate discoveries of critical therapeutic targets and strategies for neurodegenerative diseases.