Helicobacter pylori (H. pylori)
Helicobacter pylori (H. pylori) infects 50% of the world’s population and can lead to peptic ulcer disease and gastric cancer. In fact, H. pylori is the single most important risk factor for the development of gastric cancer. To cause a chronic infection, H. pylori must possess mechanisms that allow it to evade host responses. The Jones laboratory is focused on determining these mechanisms and how they may be related to disease in particular gastric carcinogenesis.
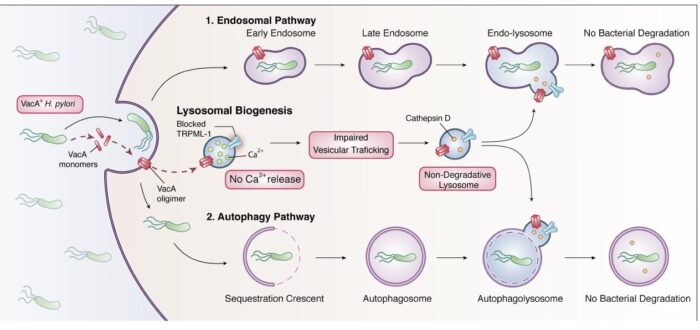
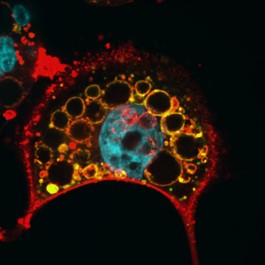
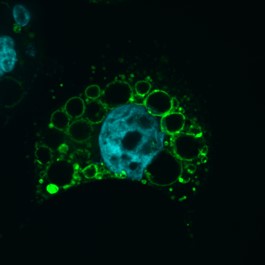
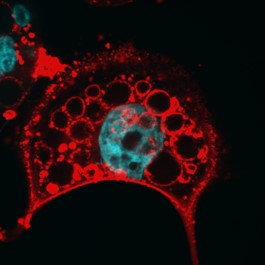
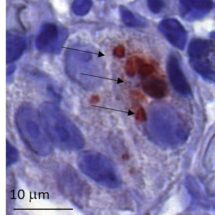
We are currently investigating how the Vacuolating cytotoxin (VacA), a virulence factor secreted by H. pylori, alters host cell trafficking to develop an intracellular niche for the bacteria where it can survive in parietal cells and escape from antibiotic eradication therapy. We have determined that VacA inhibits a lysosomal calcium channel TRPML1 to create this niche. Furthermore, activating TRPML1 eliminates the niche and kills the bacteria. We are now interested in targeting this pathway as a potential novel therapeutic. We are also interested in how inhibition of this channel can alter parietal cell function to promote disease such as gastric cancer.