Research >
Wet Lab

In Ito Lab our primary focus is to study the mechanisms and extent to which drugs are transferred into breast milk. With this information, our team seeks to reliably determine the safety of drug use in breastfeeding mothers.
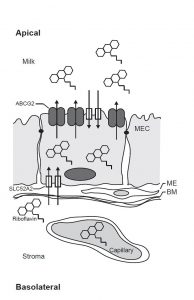 Human milk is a rich source of vitamins and nutrients for infants. While the benefits of breastfeeding for both mother and child are well documented, many women avoid breastfeeding due to the uncertainty surrounding the risk posed by many drugs, which may enter breast milk through either passive or active transporter-mediated mechanisms. However, the transport mechanisms responsible for transporting both essential nutrients into breast milk, and consequently xenobiotics have not been well elucidated. Perhaps the most important transporter for both nutrient and drug transfer into breast milk is Breast Cancer Resistance Protein (BCRP), or ABCG2. ABCG2, which is upregulated and apically localized in the lactating mammary gland, has been shown to play an important role for riboflavin (vitamin B2) excretion into breast milk, a vital vitamin for infants with breast milk being their only source. While the efflux of riboflavin into breast milk via ABCG2 has been characterized, the initial uptake of riboflavin into MECs (mammary epithelial cells) is not well defined. We reported for the first time that riboflavin uptake transporters SLC52A2 and SLC52A3 are expressed in milk fat globules of human breast milk. Furthermore, we were able to show that these transporters are upregulated during lactation in the mouse mammary gland, and that mouse SLC52A2 is localized to both apical and basolateral membranes in polarized MDCK cells. Summarizing our results, we proposed a schematic model of riboflavin transport into MECs and subsequently into breast milk.
Human milk is a rich source of vitamins and nutrients for infants. While the benefits of breastfeeding for both mother and child are well documented, many women avoid breastfeeding due to the uncertainty surrounding the risk posed by many drugs, which may enter breast milk through either passive or active transporter-mediated mechanisms. However, the transport mechanisms responsible for transporting both essential nutrients into breast milk, and consequently xenobiotics have not been well elucidated. Perhaps the most important transporter for both nutrient and drug transfer into breast milk is Breast Cancer Resistance Protein (BCRP), or ABCG2. ABCG2, which is upregulated and apically localized in the lactating mammary gland, has been shown to play an important role for riboflavin (vitamin B2) excretion into breast milk, a vital vitamin for infants with breast milk being their only source. While the efflux of riboflavin into breast milk via ABCG2 has been characterized, the initial uptake of riboflavin into MECs (mammary epithelial cells) is not well defined. We reported for the first time that riboflavin uptake transporters SLC52A2 and SLC52A3 are expressed in milk fat globules of human breast milk. Furthermore, we were able to show that these transporters are upregulated during lactation in the mouse mammary gland, and that mouse SLC52A2 is localized to both apical and basolateral membranes in polarized MDCK cells. Summarizing our results, we proposed a schematic model of riboflavin transport into MECs and subsequently into breast milk.
BCRP, or ABCG2, was originally discovered as it plays a role in cancer drug resistance through active drug efflux. It also serves as a transporter at various tissues such as intestine, kidney, placenta and blood- brain barrier. The expression of ABCG2 is dramatically upregulated during lactation. Numerous mechanisms have been explored to date, but none offers a definitive explanation for upregulation of ABCG2 during lactation in mammary gland. We investigated the role of lactogenic hormone prolactin (PRL) in the regulation of ABCG2. We were able to demonstrate for the first time that PRL induces ABCG2 expression by activating the JAK2/STAT5 signaling pathway. We have shown ABCG2 to be increased in lactation, relative to whey acidic protein (WAP) and Beta-casein (CSN2), two essential proteins found in breast milk. Following lactation, we were able to show that it is decreased in involution, reaffirming its importance as an efflux transporter in lactation.
Our findings also support the involvement of MAPK and PI3K pathways in regulation of ABCG2 in the mammary epithelium during lactation. There is evidence that PRL plays an important role in breast cancer progression, and 60- 95% of human breast cancers have overexpressed PRL expression. Our findings not only aid in understanding the mechanisms involved in ABCG2 regulation, but could help us to design therapeutic strategies targeting the PRL pathway to overcome ABCG2 associated cancer drug resistance.
For further information about these clinical studies and participation opportunities, please visit Participate in Research, or contact us directly.

