Research >
Clinical Research

Health benefit of breastfeeding to both mother and infant is widely accepted. Exclusive breastfeeding is recommended at least for the first six months of life, and at present more than 90 per cent of women start breastfeeding. However, nearly 80 per cent of these breastfeeding women take medications to treat health conditions. Many of these women are advised against breastfeeding, or avoid taking necessary drugs, because there is not enough information on drug safety during breastfeeding, which results in discontinuation of breastfeeding in almost half of them. On the other hand, in some cases the drug may enter into breast milk and carry the potential risk of toxicity to the infant. So, it is important to provide drug safety information during breastfeeding.
Our goal is to set up a “drug-in-milk” monitoring system for nursing women who are prescribed and using medications, and improve health of nursing mothers and their babies through increasing maternal adherence to drug therapy during breastfeeding, as well as establishment of breastfeeding.
This study is a continuation of “Drugs in Lactation” Analysis Consortium (DLAC).
Breastfeeding is beneficial to both mother and baby. Breastfeeding can reduce risk of infections as well as other diseases, such as diabetes for infants. However, many breastfeeding women are affected by health conditions and need to take medications during breastfeeding, some of which may enter into breast milk with a potential risk of toxicity to the infant. Sometimes, concerns about transfer of drugs through breast milk to the infants lead the mothers to either avoid breastfeeding or stop their medication.
Our goal is to measure concentration of certain drugs of interest in mother’s body fluids (such as breast milk and/or blood) when they take those medications, to develop a novel simulation model that enables us to predict and determine safety of those drugs in lactating mothers and their breastfed infants. The results of this study can help physicians and mothers to make informed decisions about taking prescribed medications during breastfeeding. Furthermore, we want to explore how individual differences in genes may affect the movement and breakdown of the drugs of interest in the body. Therefore, this study includes genetic testing to look for specific gene variations that are known to be related to the breakdown of those drugs.
Who do we study?
A nursing mother taking one of the study medications (for inquiry about our study drugs please contact us).
What is involved in this study?
We will measure drug concentration in breast milk and blood samples by pharmacokinetic (PK) testing. PK results from this study will help us understand how much of the study drug is in blood and breastmilk.
We will use this information to create a new model of drug study for breastfeeding mothers and their infants. This model will be designed to predict the amount of the drugs in breast milk of the exposed women, as well as their infant’s blood.
Participating mothers will be asked to provide one or more of the following biological samples:
- Breast Milk
- Blood from mother and/or infant- (Optional)
- Saliva from mother (Optional)
What do you need to do?
- Collect 4 samples of breast milk 5-10 ml (1-2 teaspoon) each, There is no fixed schedule for milk collection. The first sample should be collected before taking the medication, while you may collect samples 2-4 at separate time-points after taking the medication and before the next dose, at your convenience.
- Fill in a questionnaire
- Collect a saliva sample or provide a blood sample (Optional)
You may collect samples at home. The study team will send you the study package including sample collection supplies and also arrange sample pick up via pre-paid courier service. The blood sample will be collected at SickKids Phlebotomy Clinic. We will reimburse participant for parking or transportation expenses to the SickKids hospital.
Breastfeeding is beneficial to both mother and baby. However, many breastfeeding women are affected by long-term health conditions and need to take medications during breastfeeding. Sometimes, concerns about transfer of drugs through breast milk to the infants leads mothers to either avoid breastfeeding or stop their medication.
Inflammatory bowel disease (IBD) is a chronic condition that often affects women during their child bearing years. IBD is associated with an abnormal function of immune system in our body, and high levels of certain proteins that cause inflammation (e.g. Tumor Necrosis Factor alpha or TNFα). New treatments called “biologics” can target and stop these proteins from causing inflammation.
In fact, TNFα and similar proteins are also present variably in breast milk of healthy mothers and may play a role in development of infant’s brain and immune system. For this reason, it is important to learn more about TNFα concentration, as well as its relation with biologics in breast milk of women with IBD.
In this study, we examine concentrations of inflammatory proteins like TNFα in breast milk of healthy mothers and women with IBD, as well as the interaction between TNFα and biologics in breast milk of mothers with IBD. We also investigate the potential role of TNFα and biologics in development of infant learning and memory function. Participation of healthy breastfeeding mothers is an important aspect of our study, and helps us achieve better results through comparing the study findings between two groups.
With our research, we hope to provide information that is necessary for evaluating safety of biologics for infants, when they are exposed via breast milk.
We enroll two groups of participants in our study:
- Pregnant, or lactating mothers with IBD early after delivery (in the first two months after childbirth)
- Healthy pregnant, or breastfeeding mothers early after delivery (in the first two months after childbirth)
Study Visits:
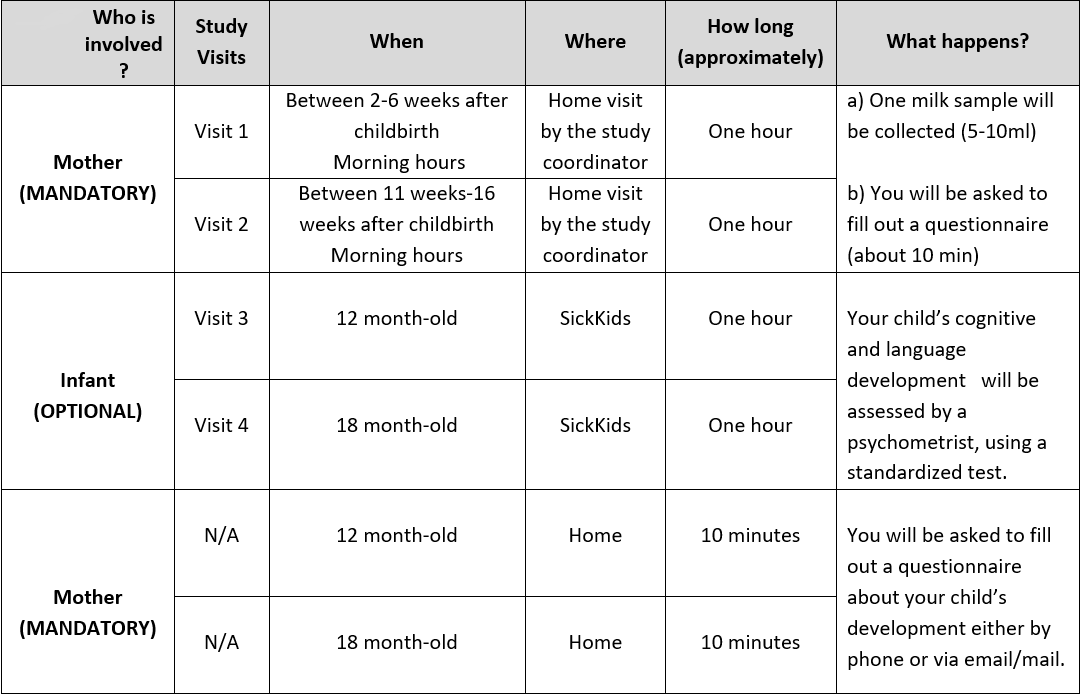
Sub-study for mothers with IBD
If you have IBD and take biologics (Infliximab or Adalimumab), you are invited to participate in the pharmacokinetic component of the study with the purpose of measuring drug levels in your milk samples. If you agree to take part in the sub-study, we will ask you to collect six milk samples, 5 ml (or one teaspoonful) each, between two doses of your medication. We provide the milk containers and you will be able to collect milk samples at home. The samples will be picked up from your home, at your convenience.
Participants will receive the memory evaluation report of the infant, and a gift card in appreciation of their time and effort. We will also reimburse participant for parking or transportation expenses to the SickKids hospital. Please contact the study coordinator if you are interested in participating in this research study.
Side effects of acute lymphoblastic leukemia treatment may occur in many organs including brain. These side effects may involve difficulties with concentrating and school performance even after the treatment ends. These seem to be common consequences of the treatment and can be debilitating, because the outcomes of academic and social difficulties may affect the child’s potential. These symptoms may vary from more to less severe among patients. Almost 25 per cent of childhood leukemia survivors who received chemotherapy have signs of these central nervous system side effects. We think that the severity of the side effects depends on differences in patient’s genes, and environmental factors, such as what food we eat. If we identify the genes and the environmental factors, then we may be able to come up with a plan for prevention and treatment of these brain side effects and possibly improve the quality of life after diagnosis and treatment.
Treatment for childhood cancer has improved tremendously over the past several decades and approximately 95 per cent of leukemia patients will be cured. Unfortunately, cancer treatment in children may have long lasting effects on brain development and may affect the ability to concentrate on tasks and remember events and assignments. We use structural Magnetic Resonance Imaging (MRI) to take a close-up look at the differences between children who have been treated for cancer and those who have not been exposed to cancer treatment. There may be subtle differences in certain regions of the brain between leukemia survivors and those that have not been treated for leukemia. These differences may further be linked to cognitive and behavioral difficulties. The overarching goal of our research is to find ways to improve the lives of childhood cancer survivors.
Pharmacogenetic testing may provide explanation as why some people may develop serious adverse reactions to some medications, while some people do not respond properly to certain medications. This two-year project serves as a test-bed for future use of pharmacogenetic testing, and helps us examine the clinical utility and feasibility of implementing pharmacogenetic testing service into routing paediatric care in future. The aim of this project is to identify those patients who are likely to respond to certain medications and/or those who may have a high probability of developing severe adverse drug reactions, through pharmacogenetic testing.
The Division of Clinical Pharmacology & Toxicology leads this project in collaboration with the Department of Laboratory Medicine (DPLM) and the Centre for Genetic Medicine. As part of the project scope, test reports (including a management plan and dosing recommendations) will be generated, if appropriate. Patients may be referred to the DART (Drug Allergy Reaction and Toxicology) clinic for further in depth consultations based on pharmacogenetic test results.
Adverse drug reactions (also known as ADRs or “side effects”) are a serious public health problem. Some ADRs are life threatening, and others may lead to permanent disability, treatment cessation, or reduced adherence to medications. Thus ADRs and their associated consequences are a striking failure and major problem of modern medicine. Genetic variation is one of the major contributing factors in the occurrence of significant proportion of ADRs.
The Canadian Pharmacogenomics Network for Drug Safety (CPNDS) is an innovative, national research program which is designed to prevent serious adverse drug reactions (ADRs) in children. The goal of the program is to explore genetic variations in children who experience serious ADRs, and to develop ADR predictive genetic tests. These genetic tests can predict who is likely to develop serious ADRs before a drug is given. This will allow doctors to prescribe drugs based on a patient’s genetic profile: the right drug at the right dose for each child to prevent ADRs. The use of these pharmacogenomics in clinical practice would also reduce health care costs associated with ADRs.
For further information about these clinical studies and participation opportunities, please visit Participate in Research, or contact us directly.

