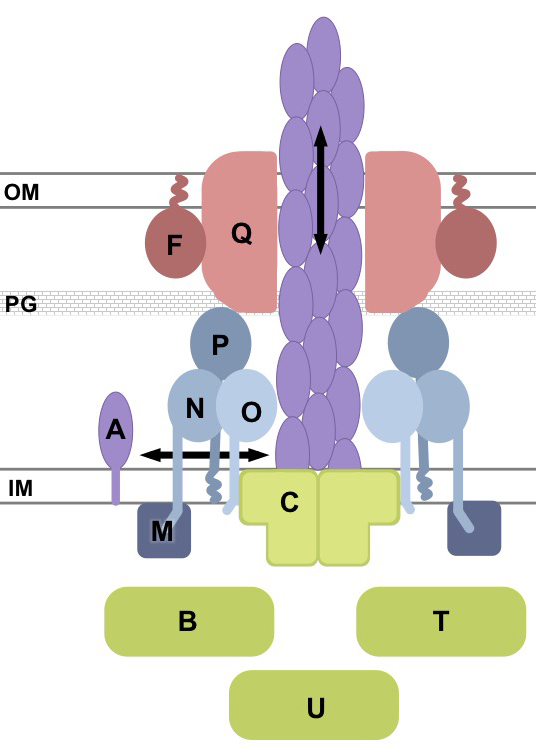
P. aeruginosa is a common gram-negative opportunistic pathogen that causes medical device infections and severe disease in immunocompromised and burn patients, as well as chronic lung infections in individuals with cystic fibrosis. Among the key virulence factors used by this organism to establish infection are its polar type IV pili (T4P). T4P are strong flexible filaments up to 4 µm in length and 50-80 Å in diameter. They are composed of thousands of copies of a single protein and mediate attachment to both living and artificial surfaces. They are also involved in bacteriophage adsorption, DNA uptake, biofilm initiation and development, and “twitching motility”, a unique form of surface-associated movement whereby in which the bacteria pull themselves rapidly towards or along a surface by retracting their T4P. Pilus retraction is capable of generating forces >100 pN, making the pilus one of the strongest nanomachines currently characterized. T4P make an attractive target for the design of novel antimicrobials as bacteria lacking T4P cannot adhere to surfaces and are therefore avirulent.
Pilus assembly is a complex process that involves over 50 proteins. In collaboration with Dr. Lori Burrows (McMaster University) we are interested in understanding how a small subset of these proteins interact to allow pilus assembly, extension, and retraction to occur. Using in vivo microbiological techniques coupled with structural biology and biochemistry we have shown that the pilus is comprised of a series of subcomplexes, that power the assembly/disassembly of the pilus (PilC plus the AAA+ ATPases PilB, and PilT), allow for its export through the outer membrane (PilQ secretin plus associated pilotin PilF), and an alignment subcomplex (PilMNOP) that connects the two.
Current projects
On going projects are focused on:
- The role of the actin paralog, PilM.
- The structure and function of the outer membrane secretin PilQ.
Selected Publications
-
The molecular mechanism of the type IVa pilus motors. McCallum M, Tammam S, Khan A, Burrows LL, Howell PL. Nat Commun. 2017 May 5;8:15091. doi: 10.1038/ncomms15091.
-
PilN binding modulates the structure and binding partners of the Pseudomonas aeruginosa Type IVa Pilus protein PilM. McCallum M, Tammam S, Little DJ, Robinson H, Koo J, Shah M, Calmettes C, Moraes TF, Burrows LL, Howell PL. J Biol Chem. 2016 Mar 28. pii: jbc.M116.718353.
-
Structure of the Pseudomonas aeruginosa Type IVa Pilus Secretin at 7.4 Å. Koo J, Lamers RP, Rubinstein JL, Burrows LL, Howell PL. Structure. 2016 Oct 4;24(10):1778-1787. doi: 10.1016/j.str.2016.08.007.
-
Functional mapping of PilF and PilQ in the Pseudomonas aeruginosa type IV pilus system. Koo J, Tang T, Harvey H, Tammam S, Sampaleanu L, Burrows LL, Howell PL. Biochemistry. 2013 Apr 30;52(17):2914-23. doi: 10.1021/bi3015345.
- PilMNOPQ from the Pseudomonas aeruginosa type IV pilus system form a transenvelope protein interaction network that interacts with PilA. Tammam S, Sampaleanu LM, Koo J, Manoharan K, Daubaras M, Burrows LL, Howell PL. J Bacteriol. 2013 May;195(10):2126-35. doi: 10.1128/JB.00032-13.