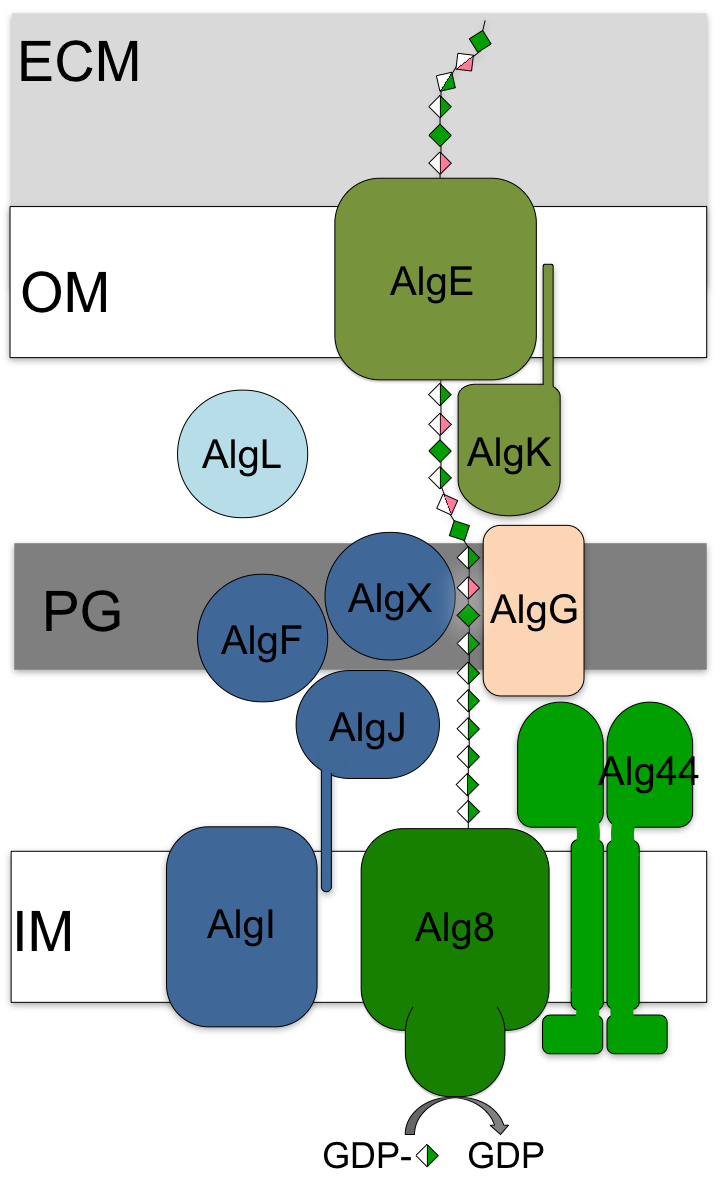
During chronic infections in the lungs of cystic fibrosis (CF) patients Pseudomonas aeruginosa converts from a non-mucoid to a mucoid phenotype. This phenotypic switch is coupled with the secretion of large amounts of the expolysaccharide alginate and is correlated with poor prognoses for these patients. Alginate is synthesized as a homopolymer of 1-4 linked ß-D-mannuronic acid, which after transport into the periplasm is chemically modified by acetylation and epimerization prior to its export from the cell. The selective acetylation of mannuronic acid helps the bacteria evade host defense mechanisms, while epimerization makes the polymer more viscous. O-acetylated alginate is also an important structural component of the biofilm, whereby acetylation increases the viscosity of the polymer and prevents degradation.
Ten proteins have been implicated in alginate polymerization, modification and export. These proteins are hypothesized to form a multi-protein secretion complex that spans both membranes (left), facilitating polymerization and transport of the polymer through the periplasm, and protecting it from degradation. We have determined the structures and functionally characterized several of the alginate biosynthesis proteins, including the outer membrane ß-barrel porin (AlgE) and scaffold lipoprotein (AlgK), and various components of the acetylation (AlgJ, AlgX) and epimerization machinery (AlgG).
Current projects
With our recent structure of the cytoplasmic PilZ domain of the inner membrane protein Alg44, current projects are focused on:
- Determining how polymerization occurs and is regulated by the bacterial second messenger c-di-GMP.
- Identifying and characterizing in vivo and in vitro the protein-protein interactions involved in secretion complex formation.
- Understanding the role of the alginate lyase (AlgL) in polymer biosynthesis.
- Determining the molecular mechanism(s) involved in alginate acetylation.
Selected Publications
- Enzymatic modifications of exopolysaccharides enhance bacterial persistence. Whitfield GB, Marmont LS, Howell PL. Front Microbiol. 2015 May 15;6:471. doi: 10.3389/fmicb.2015.00471.
- Dimeric c-di-GMP is required for post-translational regulation of alginate production in Pseudomonas aeruginosa. Whitney JC, Whitfield GB, Marmont LS, Yip P, Neculai AM, Lobsanov YD, Robinson H, Ohman DE, Howell PL. J Biol Chem. 2015 May 15;290(20):12451-62. doi: 10.1074/jbc.M115.645051.
- P. aeruginosa SGNH hydrolase-like proteins AlgJ and AlgX have similar topology but separate and distinct roles in alginate acetylation. Baker P, Ricer T, Moynihan PJ, Kitova EN, Walvoort MT, Little DJ, Whitney JC, Dawson K, Weadge JT, Robinson H, Ohman DE, Codée JD, Klassen JS, Clarke AJ, Howell PL. PLoS Pathog. 2014 Aug 28;10(8):e1004334. doi: 10.1371/journal.ppat.1004334.