Ectodermal remodelling
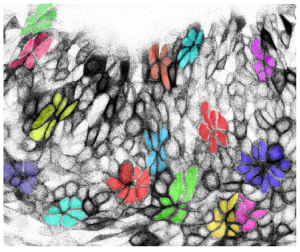
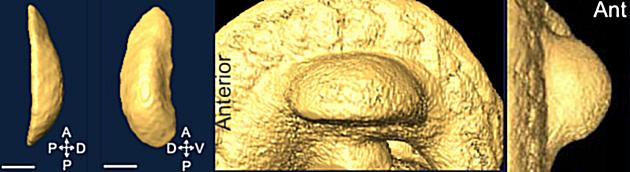
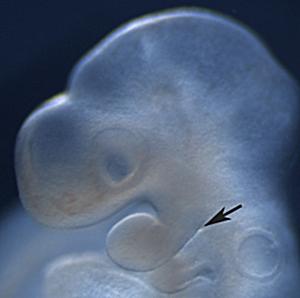
Ectoderm is an epithelial layer that becomes distinct during gastrulation. Early embryonic appendages and organ primordia are commonly surrounded by a single cell layer of ectodermal cells. Interestingly, cells within these sheets move about and exchange places with their neighbours in 2D.
Cell rearrangements accomodate rapid addition of new cells and control the shape of ectodermal tissue over time. These rearrangements are the combined result of cell-intrinsic and -extrinsic forces. Individual cells control how tightly they adhere to their neighbours at junctions and how they contract actomyosin networks that affect cell shapes and movements. The behaviours of individual cells influences tissue forces that then feed back upon cells to modify their movements. Our aim is to define how specific pathways influence the physical properties of individual cells and the transduction of force between cells.