Cell Death
One long-standing interest of the lab is understanding the mechanisms by which multicellular organisms regulate cell death and survival. We use DNA damaging agents that are commonly used to treat cancer patients as tools to investigate how cells execute a process called programmed cell death (apoptosis). We use the nematode worm Caenorhabditis elegans as a model to study apoptosis because it possesses a conserved DNA damage checkpoint, apoptosis pathway (which was first discovered in this organism), and is amenable to genetic manipulation. Equally important is the fact that this animal is multicellular, which allows us to study how various tissues and cell types influence the survival and death of their neighbours.
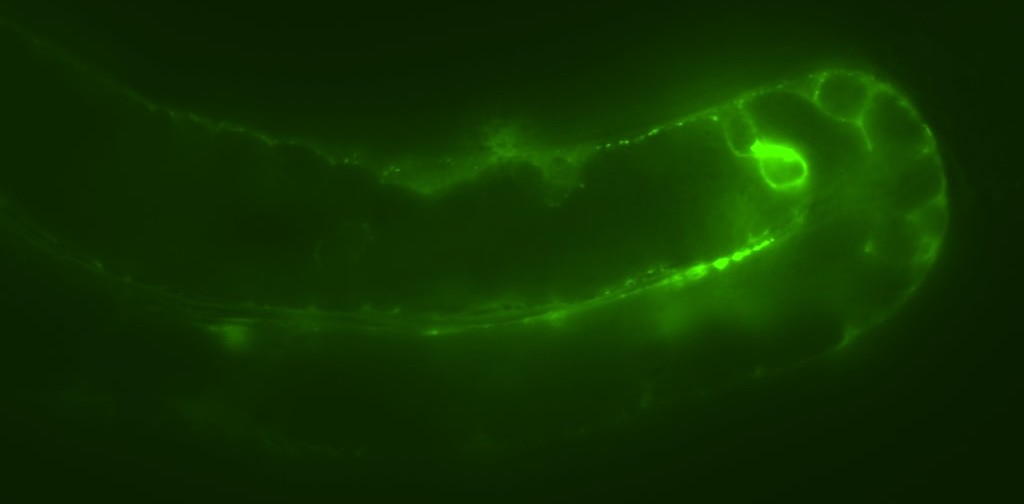
We have a historical interest in tumour suppressor p53 (CEP-1 in C. elegans), the most frequently mutated gene in human cancer, which functions to activate apoptosis or initiate repair of damaged DNA. Our work has uncovered p53-dependent and p53-independent pathways that control apoptosis, which we are exploring as potential therapeutic avenues for treating diseases such as cancer. Our lab also discovered a novel mechanism by which CEP-1 promotes fidelity of DNA repair in the reproductive tissue (germline) by suppressing non-homologous end-joining (NHEJ), an error-prone mode of DNA repair. This work has led to an interest in understanding how signalling thresholds are established to determine whether a cell undergoes apoptosis or repairs the damage to survive.
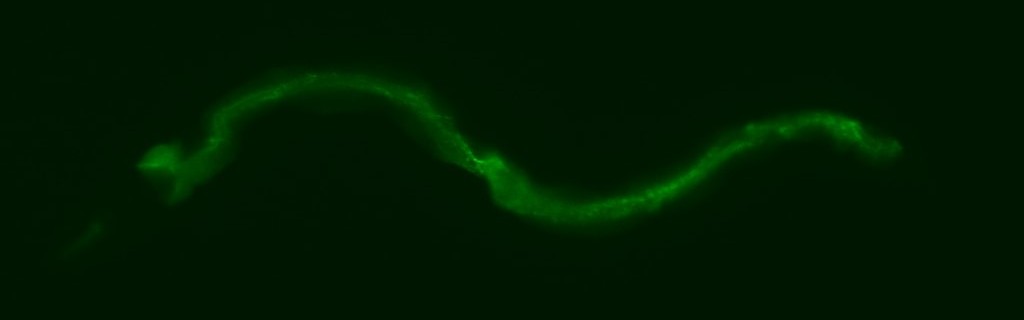
Cell Nonautonomy
We discovered a novel role for a scaffold protein called KRI-1, (KRIT1/CCM1 in humans), which is required in somatic tissue (intestine) to activate DNA damage-induced apoptosis in the germline (Ito et al., 2010). Using the tools of genetics and proteomics we delineated the signaling pathway downstream of KRI-1 (as well as its binding partners) that transduces signals across tissue boundaries to licence germ cells competent to undergo apoptosis in response to DNA damage. Future work will test for conserved mechanisms in vertebrate systems, such as zebrafish through collaborations with our partners on this project (Ian Scott, Anne-Claude Gingras, Salim Seyfried, Elizabeth Tournier-Lasserve, and Issam Awad). We are also interested in other roles for KRI-1 in development and stress response that we plan to interrogate in parallel to our work on apoptosis.
Former PhD candidate, Eric Chapman, has identified genes in the ERK5 pathway and zinc storage that function downstream of kri-1 to regulate germline apoptosis. He has shown that inactivation of KRI-1 causes defects in zinc storage that culminate in apoptosis defects, which appears to be conserved in vertebrates. Future studies will examine the role of zinc in apoptosis.

