Cerebral Cavernous Malformations
Cerebral Cavernous Malformations (CCM) is a neurovascular disease affecting up to 0.5% of the population. CCM are mulberry-like lesions found primarily in blood capillaries of the brain that are thought to be the result of junctional defects between endothelial cells lining the capillaries. This causes blood to leak into the brains of CCM patients and can cause symptoms that range from headaches and seizures to hemorrhagic stroke. Unfortunately, there are currently no pharmacological treatments for this disease, leaving invasive neurosurgery the sole treatment option.
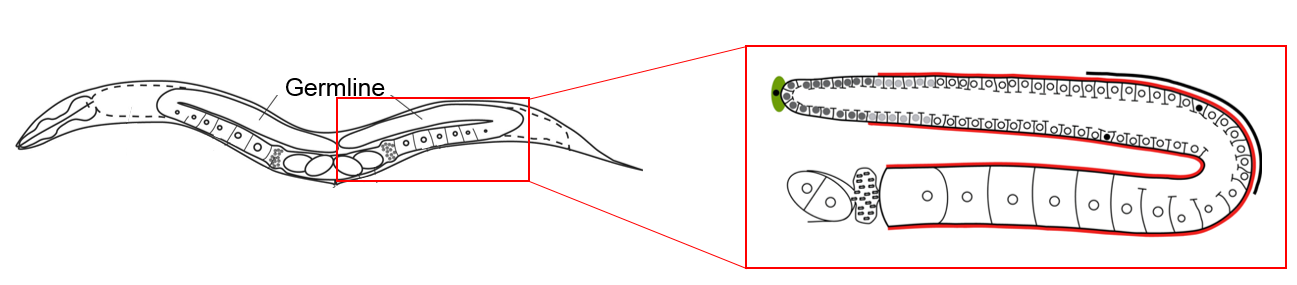
Familial CCM follows an autosomal dominant inheritance pattern caused by loss-of-function mutations in one of three genes: CCM1/KRIT-1, CCM2/Malcavernin and CCM3/PDCD10. To study the biological functions of these genes, which encode three different scaffold proteins, we use the nematode worm Caenorhabditis elegans as an in vivo model system. Our work has revealed mechanistic insight into how the CCM1 and CCM3 proteins regulate cross-tissue signalling and vascular integrity.
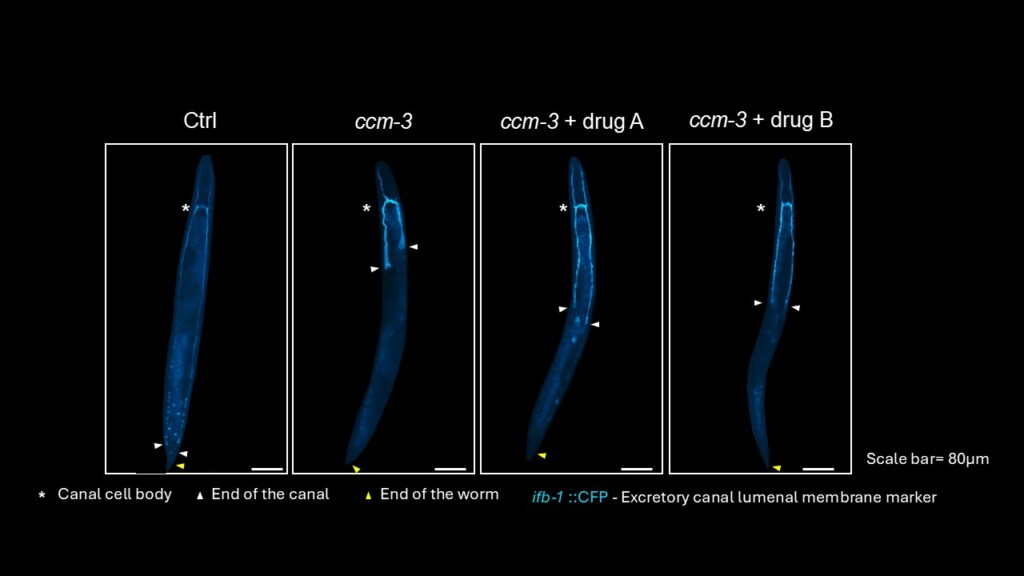
 Chien-Hui (PhD candidate) is scrutinizing the role of oxidative stress in the pathogenesis of CCM and scoliosis in collaboration with the Ciruna lab. Chien-Hui is also working on testing the potential additive effects of drug candidates for CCM using C.elegans excretory canal as a model.
Chien-Hui (PhD candidate) is scrutinizing the role of oxidative stress in the pathogenesis of CCM and scoliosis in collaboration with the Ciruna lab. Chien-Hui is also working on testing the potential additive effects of drug candidates for CCM using C.elegans excretory canal as a model.
 Naomi (Research student) is trying to determine the role of actin branching protein, fln-2 (filamin-2), in maintaining excretory canal integrity. In an unpublished CCM-3 and its binding partner GCK-1 TurboID screen, we have determined fln-2 as one of the overlapping top hits, and mutations in this gene causes excretory canal truncations similar to mutations in ccm-3. Naomi is trying to determine whether fln-2 functions downstream of ccm-3, using CRISPR technology in engineering mutants and reporters for epistatic analysis, IP-MS and RNAi screens.
Naomi (Research student) is trying to determine the role of actin branching protein, fln-2 (filamin-2), in maintaining excretory canal integrity. In an unpublished CCM-3 and its binding partner GCK-1 TurboID screen, we have determined fln-2 as one of the overlapping top hits, and mutations in this gene causes excretory canal truncations similar to mutations in ccm-3. Naomi is trying to determine whether fln-2 functions downstream of ccm-3, using CRISPR technology in engineering mutants and reporters for epistatic analysis, IP-MS and RNAi screens.
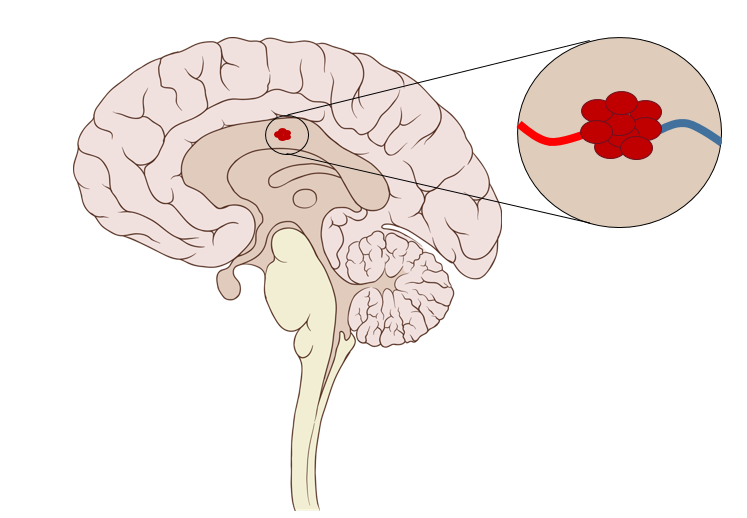
This project has been highly collaborative involving many scientists in Toronto (Ian Scott, Anne-Claude Gingras and Peter Roy), Berlin (Salim Seyfried), and Paris (Elisabeth Tournier-Lasserve). Our team is also sequencing the genomes of CCM patients to help identify additional genes that modify the spectrum and severity of this disease.
 Sam (PhD candidate) is trying to delineate where the kri-1 (CCM1 in human) and ccm-3 (CCM3) pathways converge to maintain viability of the organism. He is also working on characterizing the function of worm ccm-2 (CCM2 in human). He is also collaborating with Tony to screen human cells for genetic interactions with the CCM genes. Deiriai (Project student) and Stacey (Assistant Research Technologist) are also assisting Sam and Tony with various ccm-3 suppressor screens.
Sam (PhD candidate) is trying to delineate where the kri-1 (CCM1 in human) and ccm-3 (CCM3) pathways converge to maintain viability of the organism. He is also working on characterizing the function of worm ccm-2 (CCM2 in human). He is also collaborating with Tony to screen human cells for genetic interactions with the CCM genes. Deiriai (Project student) and Stacey (Assistant Research Technologist) are also assisting Sam and Tony with various ccm-3 suppressor screens.
 In collaboration with Charlie Boone’s lab at the Donnelley Centre in Toronto, Tony (PhD candidate) is using human cells to conduct CRISPR screens to identify genes that exhibit negative interactions with CCM1, CCM2 and CCM3. Tony is also working with Sam to investigate the excretory canal functions of genes identified in C. elegans that exhibit negative interactions with kri-1 (CCM1).
In collaboration with Charlie Boone’s lab at the Donnelley Centre in Toronto, Tony (PhD candidate) is using human cells to conduct CRISPR screens to identify genes that exhibit negative interactions with CCM1, CCM2 and CCM3. Tony is also working with Sam to investigate the excretory canal functions of genes identified in C. elegans that exhibit negative interactions with kri-1 (CCM1).

