Transgenerational Epigenetic Inheritance of Amyloids
Transgenerational epigenetic inheritance is a phenomenon that extends beyond traditional genetic transmission, allowing acquired traits and environmental influences to be passed down from one generation to the next. Unlike classical genetics, which relies on DNA to ferry information across generations, transgenerational epigenetic inheritance is understood to involve the transmission of alternative information-carrying vectors. These epigenetic signals can take the form of covalent modifications to the DNA and its associated proteins as well as the direct transmission of cytosolic protein aggregates. These signals can be altered and transmitted in response to environmental exposures, lifestyle factors, or other external influences, and they can influence gene expression patterns in ways that persist across multiple generations.
Our research is focused on unraveling the intricate mechanisms underlying the role of cytosolic protein aggregates in the transmission of epigenetic information within the model organism C. elegans. Although an established role for prion and prion-like proteins exists for model organisms such as S. cerevisiae, and cytosolic aggregates are recognized to be indispensable for mammalian oocyte competency, our understanding of the precise functions of these cytosolic structures remains incomplete. Our research aims to bridge this knowledge gap and advance our comprehension of the mechanisms and function of cytosolic aggregates in the transmission of developmental information across generations.
Cytosolic Aggregates in C. elegans
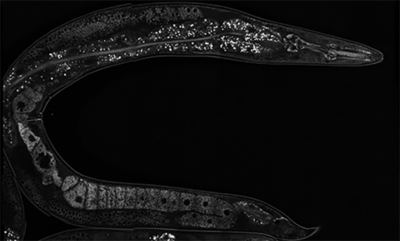
In the hermaphroditic roundworm C. elegans, the production of gametes is sequential, where the germline initially produces a finite number of sperm and then switches to oogenesis, resulting in the capacity to produce a specified number of self-progeny. Previously, former PhD student Dr. Matthew Eroglu discovered two paralogous RNA-binding proteins whose loss resulted in the multigenerational self-sterile temperature regulated (MSTR) phenotype. This phenotype causes a premature shift to oogenesis in a temperature-dependent manner as a result of an accumulation of a cytoplasmic aggregate throughout the germline, thereby transforming the germlines of these mutants to female over several generations. These aggregates have been termed “herasomes”. Although ectopic accumulation of the herasome results in the MSTR phenotype, we can see these aggregates in a wild-type context where they are stably inherited. In the future, we hope to define the composition and function of herasomes to untangle the mechanisms by which they modulate signaling cascades that alter cell fate specification in the germline and the soma.
 Autumn (PhD candidate) is interested in the transgenerational inheritance of epigenetic traits. They study the herasome, an amyloid structure whose accumulation results in the feminization of the C. elegans germline (mitotic cells that would normally be bound to develop into sperm instead develop into oocytes). Using biochemistry techniques and high-resolution electron microscopy, they are studying the mechanisms through which the herasome influences germline sex determination.
Autumn (PhD candidate) is interested in the transgenerational inheritance of epigenetic traits. They study the herasome, an amyloid structure whose accumulation results in the feminization of the C. elegans germline (mitotic cells that would normally be bound to develop into sperm instead develop into oocytes). Using biochemistry techniques and high-resolution electron microscopy, they are studying the mechanisms through which the herasome influences germline sex determination.
Alternative Polyadenylation
Rhea‘s (PhD candidate) study employs neuroblastoma cell lines and single-cell RNA sequencing (scRNAseq) to investigate the role of alternative polyadenylation (APA) in cancer. APA is a critical RNA processing step with a range of implications, including mRNA stability, mRNA and protein localization, and translational efficiency. Over 60% of human genes harbour multiple polyadenylation sites which can result in transcripts with extensive 3’UTR length variations through APA.
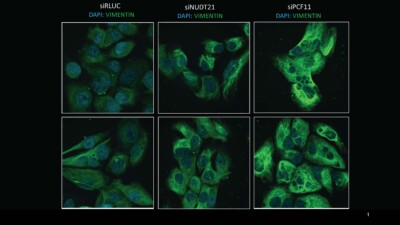
 Recent findings underscore APA’s synergy with distinct oncogenes in adult cancers. Previous work from the Derry lab using colorectal and pancreatic cancer cells with KRAS gain-of-function mutations as the model system, showed that the shortening of 3’utrs of specific genes upon NUDT21 knockdown increased invasiveness and enhanced the epithelial-to-mesenchymal transition (EMT). Leveraging functional assays such as transwell migration assays and immunofluorescence imaging, along with scRNAseq data, Rhea is looking at the potential involvement of APA in cell state transitions, analogous to EMT within paediatric cancers, using neuroblastoma as a paradigm. Neuroblastoma, like most pediatric cancers, shows a paucity in somatic alterations. Therefore, investigating the impact of APA on cell state alterations in neuroblastoma stands to enhance our comprehension of APA-driven post-transcriptional reprogramming in paediatric cancers. This approach will help us elucidate biological pathways and novel therapeutic targets in neuroblastoma. Furthermore, delving into APA’s role in neuroblastoma metastasis will yield insights into how cancers exploit APA to advance disease progression.
Recent findings underscore APA’s synergy with distinct oncogenes in adult cancers. Previous work from the Derry lab using colorectal and pancreatic cancer cells with KRAS gain-of-function mutations as the model system, showed that the shortening of 3’utrs of specific genes upon NUDT21 knockdown increased invasiveness and enhanced the epithelial-to-mesenchymal transition (EMT). Leveraging functional assays such as transwell migration assays and immunofluorescence imaging, along with scRNAseq data, Rhea is looking at the potential involvement of APA in cell state transitions, analogous to EMT within paediatric cancers, using neuroblastoma as a paradigm. Neuroblastoma, like most pediatric cancers, shows a paucity in somatic alterations. Therefore, investigating the impact of APA on cell state alterations in neuroblastoma stands to enhance our comprehension of APA-driven post-transcriptional reprogramming in paediatric cancers. This approach will help us elucidate biological pathways and novel therapeutic targets in neuroblastoma. Furthermore, delving into APA’s role in neuroblastoma metastasis will yield insights into how cancers exploit APA to advance disease progression.
 Symon (Research student) is investigating the role of mml-1 (myc and mondo-like-1), the C. elegans homologue of MLXIP, in regulating vulva development in oncogenic let-60 (Ras) mutant strains. The Ras/MAPK pathway is implicated in various cancers, and Alternative Polyadenylation (APA), a process that regulates the length of 3’UTRs in gene transcripts, has been shown to modulate Ras signalling. In a previous screen conducted in our lab to identify targets of cfim-1, an APA factor, mml-1 was found to act as a key regulator that buffers oncogenic Ras activity. In particular, ablation of mml-1 was shown to suppress the multivulva (Muv) phenotype in Ras mutants. Symon is working to determine how mml-1 modulates Ras signalling and interacts with regulatory pathways, with the aim of uncovering novel mechanisms for controlling oncogenic Ras activity.
Symon (Research student) is investigating the role of mml-1 (myc and mondo-like-1), the C. elegans homologue of MLXIP, in regulating vulva development in oncogenic let-60 (Ras) mutant strains. The Ras/MAPK pathway is implicated in various cancers, and Alternative Polyadenylation (APA), a process that regulates the length of 3’UTRs in gene transcripts, has been shown to modulate Ras signalling. In a previous screen conducted in our lab to identify targets of cfim-1, an APA factor, mml-1 was found to act as a key regulator that buffers oncogenic Ras activity. In particular, ablation of mml-1 was shown to suppress the multivulva (Muv) phenotype in Ras mutants. Symon is working to determine how mml-1 modulates Ras signalling and interacts with regulatory pathways, with the aim of uncovering novel mechanisms for controlling oncogenic Ras activity.