Publications
High impact and peer-reviewed. Check out our latest published research.
Publications
High impact and peer-reviewed. Check out our latest published research.
Featured Articles
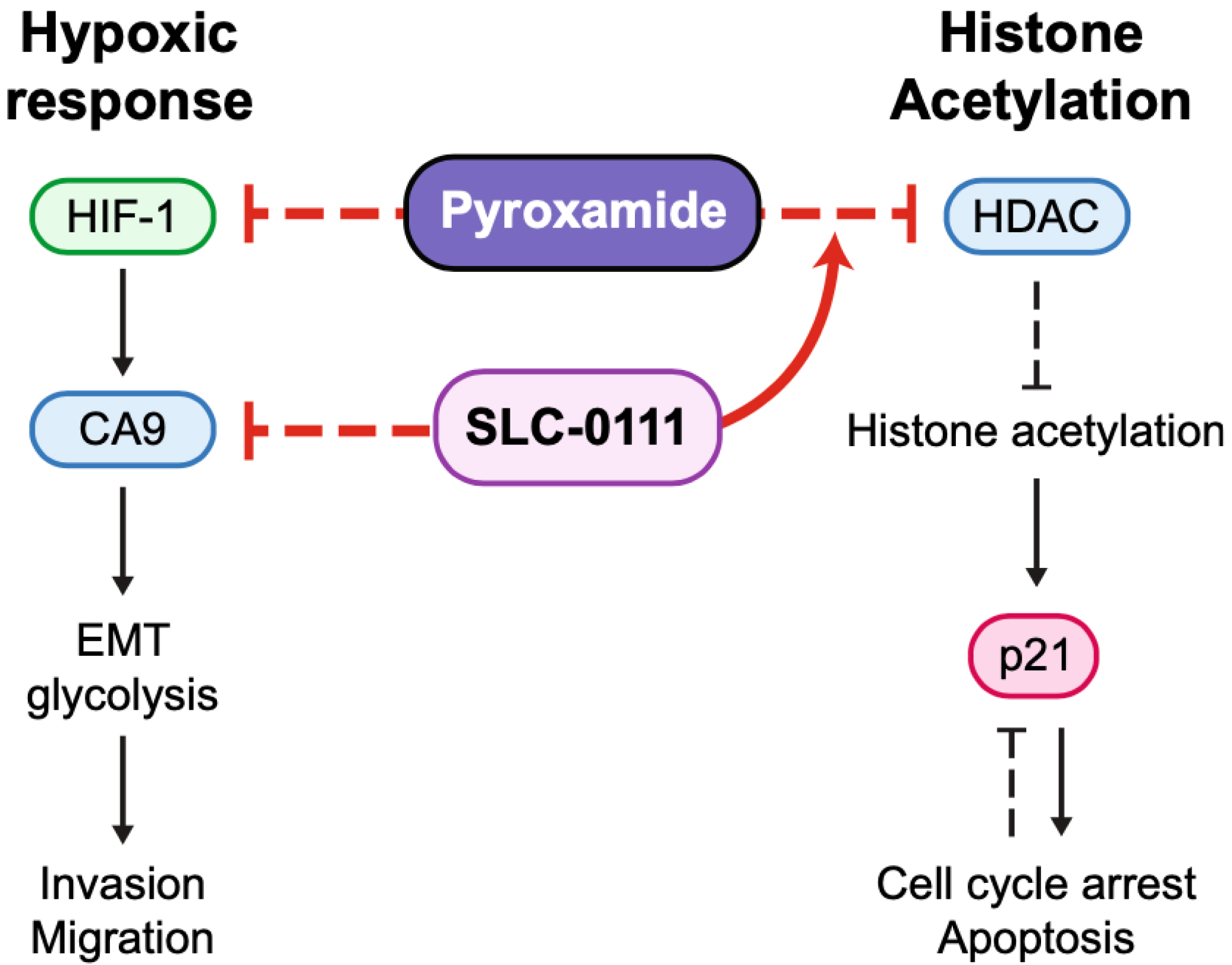
Combination treatment with histone deacetylase and carbonic anhydrase 9 inhibitors shows therapeutic potential in experimental diffuse intrinsic pontine glioma
Naohide Fujita, Andrew Bondoc, Sergio Simoes, Joji Ishida, Michael S. Taccone, Amanda Luck, Dilakshan Srikanthan, Robert Siddaway, Adrian Levine, Nesrin Sabha, Stacey Krumholtz, Akihide Kondo, Hajime Arai, Christian Smith, Paul McDonald, Cynthia Hawkins, Shoukat Dedhar, James Rutka
Brain Tumor Pathology, Published online: 24 September 2024
Diffuse intrinsic pontine glioma (DIPG) remains a significant therapeutic challenge due to the lack of effective and safe treatment options. This study explores the potential of combining histone deacetylase (HDAC) and carbonic anhydrase 9 (CA9) inhibitors in treating DIPG. Analysis of RNA sequencing data and tumor tissue from patient samples for the expression of the carbonic anhydrase family and hypoxia signaling pathway activity revealed clinical relevance for targeting CA9 in DIPG. A synergy screen was conducted using CA9 inhibitor SLC-0111 and HDAC inhibitors panobinostat, vorinostat, entinostat, and pyroxamide. The combination of SLC-0111 and pyroxamide demonstrated the highest synergy and was selected for further analysis. Combining SLC-0111 and pyroxamide effectively inhibited DIPG cell proliferation, reduced cell migration and invasion potential, and enhanced histone acetylation, leading to decreased cell population in S Phase. Additionally, the combination therapy induced a greater reduction in intracellular pH than either agent alone. Data from this study suggest that the combination of SLC-0111 and pyroxamide holds promise for treating experimental DIPG, and further investigation of this combination therapy in preclinical models is warranted to evaluate its potential as a viable treatment for DIPG.
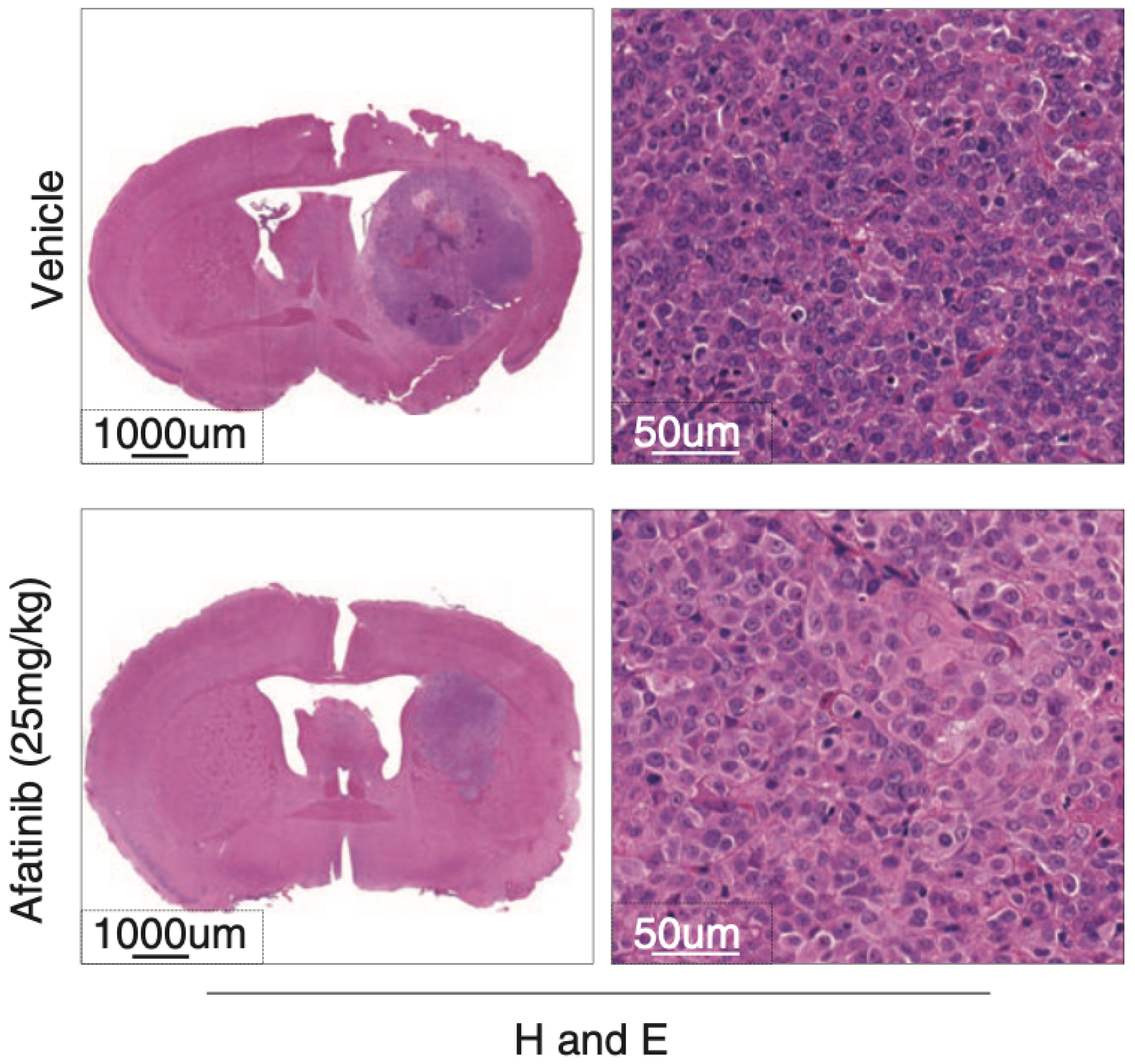
A kinome drug screen identifies multi-TKI synergies and ERBB2 signaling as a therapeutic vulnerability in MYC/TYR subgroup atypical teratoid rhabdoid tumors
Brian Golbourn, Ben Ho, Andrew Bondoc, Amanda Luck, Xiaolian Fan, Elizabeth Richardson, Richard Marcellus, Michael Prakesch, Mathew Halbert, Nishant Agrawal, Christian Smith, Annie Huang, James T. Rutka
Neuro-Oncology, XX(XX), 1–17 (2024)
Atypical teratoid rhabdoid tumor (ATRT) is a rare, devastating, and largely incurable pediatric brain tumor. Although recent studies have uncovered 3 molecular subgroups of ATRTs with distinct disease patterns, and signaling features, the therapeutic profiles of ATRT subgroups remain incompletely elucidated. We examined the effect of 465 kinase inhibitors on a panel of ATRT subgroup-specific cell lines. We then applied multiomics analyses to investigate the underlying molecular mechanism of kinase inhibitor efficacy in ATRT subgroups. We observed that ATRT cell lines are broadly sensitive to inhibitors of the PI3K and MAPK signaling pathways, as well as CDKs, AURKA/B kinases, and polo-like kinase 1. We identified 2 classes of multikinase inhibitors predominantly targeting receptor tyrosine kinases including PDGFR and EGFR/ERBB2 in MYC/TYR ATRT cells. The PDGFRB inhibitor, Dasatinib, synergistically affected MYC/TYR ATRT cell growth when combined with broad-acting PI3K and MAPK pathway inhibitors, including Rapamycin and Trametinib. We observed that MYC/TYR ATRT cells were also distinctly sensitive to various inhibitors of ERBB2 signaling. Transcriptional, H3K27Ac ChIPSeq, ATACSeq, and HiChIP analyses of primary MYC/TYR ATRTs revealed ERBB2 expression, which correlated with differential methylation and activation of a distinct enhancer element by DNA looping. Significantly, we show the brain penetrant EGFR/ERBB2 inhibitor, Afatinib, specifically inhibited in vitro and in vivo growth of MYC/TYR ATRT cells. Taken together, our studies suggest combined treatments with PDGFR and ERBB2-directed TKIs with inhibitors of the PI3K and MAPK pathways as an important new therapeutic strategy for the MYC/TYR subgroup of ATRTs.
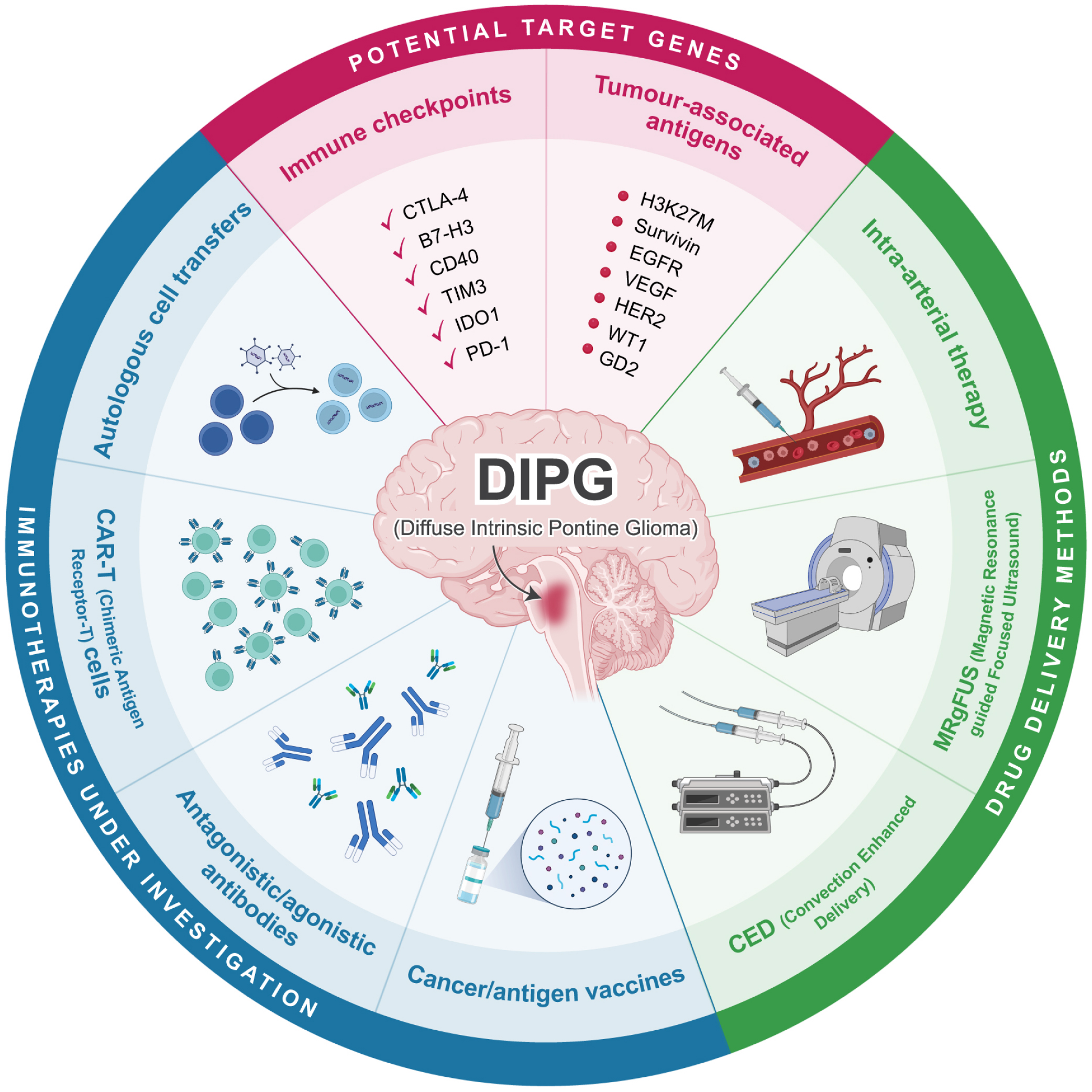
Current immunotherapeutic approaches to diffuse intrinsic pontine glioma
Catherine Lin, Christian Smith and James Rutka
Frontiers in Genetics, Volume 15 (2024)
Diffuse intrinsic pontine glioma (DIPG) is an aggressive brain tumour that occurs in the pons of the brainstem and accounts for over 80% of all brainstem gliomas. The median age at diagnosis is 6–7 years old, with less than 10% overall survival 2 years after diagnosis and less than 1% after 5years. DIPGs are surgically inaccessible, and radiation therapy provides only transient benefit, with death ensuing from relentless local tumour infiltration. DIPGs are now the leading cause of brain tumour deaths in children, with a societal cancer burden in years of life lost (YLL) of more than 67 per individual, versus approximately 14 and 16 YLL for lung and breast cancer respectively. More than 95 clinical drug trials have been conducted on children with DIPGs, and all have failed to improve survival. No single or combination chemotherapeutic strategy has been successful to date because of our inability to identify targeted drugs for this disease and to deliver these drugs across an intact blood-brain barrier (BBB). Accordingly, there has been an increased focus on immunotherapy research in DIPG, with explorations into treatments such as chimeric antigen receptor T (CAR-T) cells, immune checkpoint blockades, cancer vaccines, and autologous cell transfer therapy. Here, we review the most recent advances in identifying genetic factors influencing the development of immunotherapy for DIPG. Additionally, we explore emerging technologies such as Magnetic Resonance-guided Focused Ultrasound (MRgFUS) in potential combinatorial approaches to treat DIPG.
Dive Deeper.
A full list of our publications by year. Click any title to read the full article.
2024
Shahzad U, Nikolopoulos M, Li C, Johnston M, Wang JJ, Sabha N, Varn FS, Riemenschneider A, Krumholtz S, Krishnamurthy PM, Smith CA, Karamchandani J, Watts JK, Verhaak RGW, Gallo M, Rutka JT, Das S. CASCADES, a novel SOX2 super-enhancer-associated long noncoding RNA, regulates cancer stem cell specification and differentiation in glioblastoma. Mol Oncol. Epub ahead of print.
Fujita N, Bondoc A, Simoes S, Ishida J, Taccone MS, Luck A, Srikanthan D, Siddaway R, Levine A, Sabha N, Krumholtz S, Kondo A, Arai H, Smith C, McDonald P, Hawkins C, Dedhar S, Rutka J. Combination treatment with histone deacetylase and carbonic anhydrase 9 inhibitors shows therapeutic potential in experimental diffuse intrinsic pontine glioma. Brain Tumor Pathol. Epub ahead of print.
Golbourn B, Ho B, Bondoc A, Luck A, Fan X, Richardson E, Marcellus R, Prakesch M, Halbert M, Agrawal N, Smith C, Huang A, Rutka JT. A kinome drug screen identifies multi-TKI synergies and ERBB2 signaling as a therapeutic vulnerability in MYC/TYR subgroup atypical teratoid rhabdoid tumors. Neuro Oncol, 26 (10), 1895-1911.
Lin C, Smith C, Rutka J. (2024). Current immunotherapeutic approaches to diffuse intrinsic pontine glioma. Frontiers in Genetics, 15.
2021
Ishida J, Alli S, Bondoc A, Golbourn B, Sabha N, Mikloska K, Krumholtz S, Srikanthan D, Fujita N, Luck A, Maslink C, Smith C, Hynynen K, Rutka J. (2021). MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma. Journal of Controlled Release, 330, 1034–1045.
Shahzad, U., Taccone, M. S., Kumar, S. A., Okura, H., Krumholtz, S., Ishida, J., Mine, C., Gouveia, K., Edgar, J., Smith, C., Hayes, M., Huang, X., Derry, W. B., Taylor, M. D., & Rutka, J. T. (2021). Modeling human brain tumors in flies, worms, and zebrafish: From proof of principle to novel therapeutic targets. Neuro-Oncology, 23 (5), 718–731.
Srikanthan, D., Taccone, M. S., Van Ommeren, R., Ishida, J., Krumholtz, S. L., & Rutka, J. T. (2021). Diffuse intrinsic pontine glioma: Current insights and future directions. Chinese Neurosurgical Journal, 7 (6).
2020
Wong, R., Gong, H., Alanazi, R., Bondoc, A., Luck, A., Sabha, N., Horgen, F. D., Fleig, A., Rutka, J. T., Feng, Z.-P., & Sun, H.-S. (2020). Inhibition of TRPM7 with waixenicin A reduces glioblastoma cellular functions. Cell Calcium, 92, 102307.
2018
Alli, S., Figueiredo, C. A., Golbourn, B., Sabha, N., Wu, M. Y., Bondoc, A., Luck, A., Coluccia, D., Maslink, C., Smith, C., Wurdak, H., Hynynen, K., O’Reilly, M., & Rutka, J. T. (2018). Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. Journal of Controlled Release: Official Journal of the Controlled Release Society, 281, 29–41.
Bertrand, K. C., Faria, C. C., Skowron, P., Luck, A., Garzia, L., Wu, X., Agnihotri, S., Smith, C. A., Taylor, M. D., Mack, S. C., & Rutka, J. T. (2018). A functional genomics approach to identify pathways of drug resistance in medulloblastoma. Acta Neuropathologica Communications, 6 (1), 146.
Coluccia, D., Figueiredo, C. A., Wu, M. Y., Riemenschneider, A. N., Diaz, R., Luck, A., Smith, C., Das, S., Ackerley, C., O’Reilly, M., Hynynen, K., & Rutka, J. T. (2018). Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine: Nanotechnology, Biology, and Medicine, 14 (4), 1137–1148.
Diaz, R. J., Luck, A., Bondoc, A., Golbourn, B., Picard, D., Remke, M., Loukides, J., Sabha, N., Smith, C., Cusimano, M. D., & Rutka, J. T. (2018). Characterization of a Clival Chordoma Xenograft Model Reveals Tumor Genomic Instability. The American Journal of Pathology, 188 (12), 2902–2911.
2017
Lassaletta, A., Zapotocky, M., Mistry, M., Ramaswamy, V., Honnorat, M., Krishnatry, R., Guerreiro Stucklin, A., Zhukova, N., Arnoldo, A., Ryall, S., Ling, C., McKeown, T., Loukides, J., Cruz, O., de Torres, C., Ho, C.-Y., Packer, R. J., Tatevossian, R., Qaddoumi, I., … Tabori, U. (2017). Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 35 (25), 2934–2941.
2016
Coluccia, D., Figuereido, C., Isik, S., Smith, C., & Rutka, J. T. (2016). Medulloblastoma: Tumor Biology and Relevance to Treatment and Prognosis Paradigm. Current Neurology and Neuroscience Reports, 16 (5), 43.
Agnihotri, S., Golbourn, B., Huang, X., Remke, M., Younger, S., Cairns, R. A., Chalil, A., Smith, C. A., Krumholtz, S.-L., Mackenzie, D., Rakopoulos, P., Ramaswamy, V., Taccone, M. S., Mischel, P. S., Fuller, G. N., Hawkins, C., Stanford, W. L., Taylor, M. D., Zadeh, G., & Rutka, J. T. (2016). PINK1 Is a Negative Regulator of Growth and the Warburg Effect in Glioblastoma. Cancer Research, 76 (16), 4708–4719.
Okura, H., Golbourn, B. J., Shahzad, U., Agnihotri, S., Sabha, N., Krieger, J. R., Figueiredo, C. A., Chalil, A., Landon-Brace, N., Riemenschneider, A., Arai, H., Smith, C. A., Xu, S., Kaluz, S., Marcus, A. I., Van Meir, E. G., & Rutka, J. T. (2016). A role for activated Cdc42 in glioblastoma multiforme invasion. Oncotarget, 7 (35), 56958–56975.
Weeks, A., Agnihotri, S., Lymer, J., Chalil, A., Diaz, R., Isik, S., Smith, C., & Rutka, J. T. (2016). Epithelial Cell Transforming 2 and Aurora Kinase B Modulate Formation of Stress Granule-Containing Transcripts from Diverse Cellular Pathways in Astrocytoma Cells. The American Journal of Pathology, 186 (6), 1674–1687.
2015
Diaz, R. J., Golbourn, B., Faria, C., Picard, D., Shih, D., Raynaud, D., Leadly, M., MacKenzie, D., Bryant, M., Bebenek, M., Smith, C. A., Taylor, M. D., Huang, A., & Rutka, J. T. (2015). Mechanism of action and therapeutic efficacy of Aurora kinase B inhibition in MYC overexpressing medulloblastoma. Oncotarget, 6 (5), 3359–3374.
Faria, C. C., Agnihotri, S., Mack, S. C., Golbourn, B. J., Diaz, R. J., Olsen, S., Bryant, M., Bebenek, M., Wang, X., Bertrand, K. C., Kushida, M., Head, R., Clark, I., Dirks, P., Smith, C. A., Taylor, M. D., & Rutka, J. T. (2015). Identification of alsterpaullone as a novel small molecule inhibitor to target group 3 medulloblastoma. Oncotarget, 6(25), 21718–21729.
Faria, C. C., Golbourn, B. J., Dubuc, A. M., Remke, M., Diaz, R. J., Agnihotri, S., Luck, A., Sabha, N., Olsen, S., Wu, X., Garzia, L., Ramaswamy, V., Mack, S. C., Wang, X., Leadley, M., Reynaud, D., Ermini, L., Post, M., Northcott, P. A., … Rutka, J. T. (2015). Foretinib is effective therapy for metastatic sonic hedgehog medulloblastoma. Cancer Research, 75(1), 134–146.
2014
Diaz, R. J., McVeigh, P. Z., O’Reilly, M. A., Burrell, K., Bebenek, M., Smith, C., Etame, A. B., Zadeh, G., Hynynen, K., Wilson, B. C., & Rutka, J. T. (2014). Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: Potential for targeting experimental brain tumors. Nanomedicine: Nanotechnology, Biology, and Medicine, 10(5), 1075–1087.
Okura, H., Smith, C. A., & Rutka, J. T. (2014). Gene therapy for malignant glioma. Molecular and Cellular Therapies, 2, 21.
Park, J.-B., Agnihotri, S., Golbourn, B., Bertrand, K. C., Luck, A., Sabha, N., Smith, C. A., Byron, S., Zadeh, G., Croul, S., Berens, M., & Rutka, J. T. (2014). Transcriptional profiling of GBM invasion genes identifies effective inhibitors of the LIM kinase-Cofilin pathway. Oncotarget, 5(19), 9382–9395.
2013
Terakawa, Y., Agnihotri, S., Golbourn, B., Nadi, M., Sabha, N., Smith, C. A., Croul, S. E., & Rutka, J. T. (2013). The role of drebrin in glioma migration and invasion. Experimental Cell Research, 319(4), 517–528.
2012
Diaz, R. J., Golbourn, B., Shekarforoush, M., Smith, C. A., & Rutka, J. T. (2012). Aurora kinase B/C inhibition impairs malignant glioma growth in vivo. Journal of Neuro-Oncology, 108(3), 349–360.
Diaz, R. J., Guduk, M., Romagnuolo, R., Smith, C. A., Northcott, P., Shih, D., Berisha, F., Flanagan, A., Munoz, D. G., Cusimano, M. D., Pamir, M. N., & Rutka, J. T. (2012). High-resolution whole-genome analysis of skull base chordomas implicates FHIT loss in chordoma pathogenesis. Neoplasia (New York, N.Y.), 14(9), 788–798.
Etame, A. B., Diaz, R. J., O’Reilly, M. A., Smith, C. A., Mainprize, T. G., Hynynen, K., & Rutka, J. T. (2012). Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine: Nanotechnology, Biology, and Medicine, 8(7), 1133–1142.
Etame, A. B., Diaz, R. J., Smith, C. A., Mainprize, T. G., Hynynen, K., & Rutka, J. T. (2012). Focused ultrasound disruption of the blood-brain barrier: A new frontier for therapeutic delivery in molecular neurooncology. Neurosurgical Focus, 32(1), E3.
Weeks, A., Okolowsky, N., Golbourn, B., Ivanchuk, S., Smith, C., & Rutka, J. T. (2012). ECT2 and RASAL2 mediate mesenchymal-amoeboid transition in human astrocytoma cells. The American Journal of Pathology, 181(2), 662–674.
Seol, H. J., Chang, J. H., Yamamoto, J., Romagnuolo, R., Suh, Y., Weeks, A., Agnihotri, S., Smith, C. A., & Rutka, J. T. (2012). Overexpression of CD99 Increases the Migration and Invasiveness of Human Malignant Glioma Cells. Genes & Cancer, 3(9–10), 535–549.
2011
Etame, A. B., Smith, C. A., Chan, W. C. W., & Rutka, J. T. (2011). Design and potential application of PEGylated gold nanoparticles with size-dependent permeation through brain microvasculature. Nanomedicine: Nanotechnology, Biology, and Medicine, 7(6), 992–1000.
Faria, C. M. C., Rutka, J. T., Smith, C., & Kongkham, P. (2011). Epigenetic mechanisms regulating neural development and pediatric brain tumor formation. Journal of Neurosurgery. Pediatrics, 8(2), 119–132.
Restrepo, A., Smith, C. A., Agnihotri, S., Shekarforoush, M., Kongkham, P. N., Seol, H. J., Northcott, P., & Rutka, J. T. (2011). Epigenetic regulation of glial fibrillary acidic protein by DNA methylation in human malignant gliomas. Neuro-Oncology, 13(1), 42–50.
2010
Nakahara, Y., Northcott, P. A., Li, M., Kongkham, P. N., Smith, C., Yan, H., Croul, S., Ra, Y.-S., Eberhart, C., Huang, A., Bigner, D., Grajkowska, W., Van Meter, T., Rutka, J. T., & Taylor, M. D. (2010). Genetic and epigenetic inactivation of Kruppel-like factor 4 in medulloblastoma. Neoplasia (New York, N.Y.), 12(1), 20–27.
Kongkham, P. N., Northcott, P. A., Croul, S. E., Smith, C. A., Taylor, M. D., & Rutka, J. T. (2010). The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene, 29(20), 3017–3024.
Kongkham, P. N., Onvani, S., Smith, C. A., & Rutka, J. T. (2010). Inhibition of the MET Receptor Tyrosine Kinase as a Novel Therapeutic Strategy in Medulloblastoma. Translational Oncology, 3(6), 336–343.
2009
Seol, H. J., Smith, C. A., Salhia, B., & Rutka, J. T. (2009). The Guanine Nucleotide Exchange Factor SWAP-70 Modulates the Migration and Invasiveness of Human Malignant Glioma Cells. Translational Oncology, 2(4), 300–309.
Osawa, H., Smith, C. A., Ra, Y. S., Kongkham, P., & Rutka, J. T. (2009). The role of the membrane cytoskeleton cross-linker ezrin in medulloblastoma cells. Neuro-Oncology, 11(4), 381–393.
2008
Salhia, B., Hwang, J. H., Smith, C. A., Nakada, M., Rutka, F., Symons, M., & Rutka, J. T. (2008). Role of myosin II activity and the regulation of myosin light chain phosphorylation in astrocytomas. Cell Motility and the Cytoskeleton, 65(1), 12–24.
Kongkham, P. N., Northcott, P. A., Ra, Y. S., Nakahara, Y., Mainprize, T. G., Croul, S. E., Smith, C. A., Taylor, M. D., & Rutka, J. T. (2008). An epigenetic genome-wide screen identifies SPINT2 as a novel tumor suppressor gene in pediatric medulloblastoma. Cancer Research, 68(23), 9945–9953.