Nedd4 family of ubiquitin ligases
Nedd4 proteins are ubiquitin ligases of the C2-WW-Hect family. We have been studying their structure, regulation and biological functions, and identified numerous substrates for them using various proteomics approaches. We are particularly interested in the role of these family members in regulating endocytosis and vesicular sorting of transmembrane proteins, including the Epithelial Na Channel ENaC, FGFR1, LAPTM4/5, and others. We have been using both genetic and biochemical approaches in mice and other model organisms to decipher the physiological functions of Nedd4 family proteins in vivo, the biochemical regulation of their catalytic activity, and their role in human diseases, such as hypertension (eg. Liddle syndrome), cystic fibrosis, and cancer.
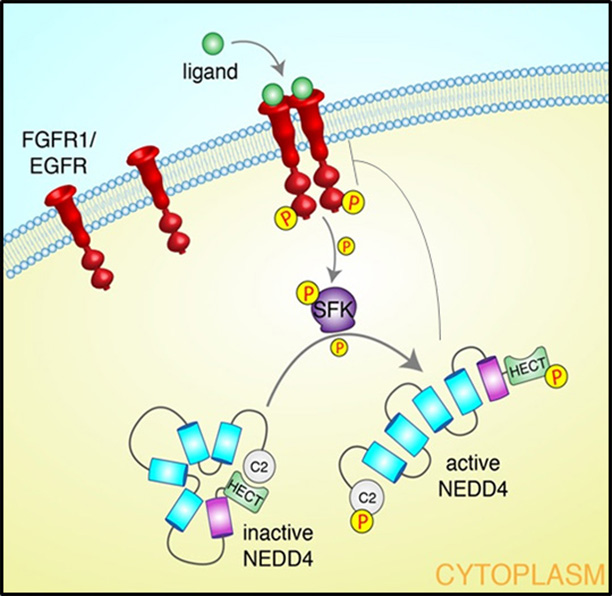
Mutual regulation of the FGFR1 and the ubiquitin ligase Nedd4.
Description of image: Nedd4 (composed of a C2-WW(n)-HECT domain architecture) binds the Fibroblasts Growth Factor Receptor, FGFR1 (a receptor Tyrosine kinase). Upon ligand (FGF) binding, the FGFR1 becomes activated (phosphorylated [P] on tyrosine residues), leading to binding to and activation of the SRC family of tyrosine kinases (SFK). SFK then phosphorylates Nedd4 on its C2 and HECT domains, releasing an inhibitory conformation of Nedd4, thus enhancing catalytic activity of this ubiquitin ligase, which now ubiquitinates the FGFR1, targeting it for endocytosis and degradation, to stop FGFR1 signaling. Thus, the active FGFR1 enhances enzymatic activity of its suppressor, Nedd4, to prevent excess FGFR1 signaling, developmental defects or cancer.
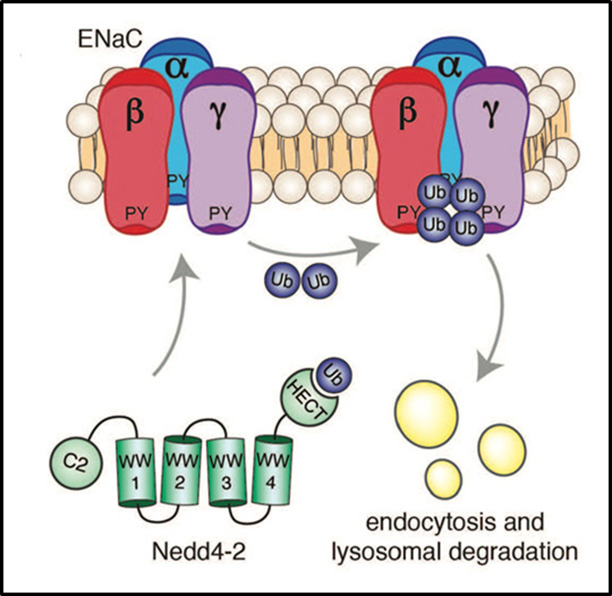
The epithelial sodium channel ENaC is suppressed by the ubiquitin ligase Nedd4-2.
Description of image: Nedd4-2, a ubiquitin ligase comprised of a C2-WW(n)-HECT domain architecture, binds ENaC, a sodium channel composed of 3 subunits: alpha-, beta- and gamma- subunits. Nedd4-2 then ubiquitinates ENaC (i.e. adds ubiquitin [Ub] to it), thus targeting this channel for endocytosis and lysosomal degradation. This stops sodium from entering cells via ENaC, to prevent excess sodium loading, hypertension, and lung dehydration.
Signal Transduction: mTORC1
We showed earlier that Nedd4 proteins regulate localization of some LAPTM4/5 family members to the lysosomal membrane, and that LAPTM4b, in turn, recruits the leucine transporter LAT1 to the lysosomal membrane to promote mTORC1 activation. mTORC1 is a master regulator of protein synthesis, cell growth and proliferation, and is critical for cancer cell survival and growth. More recently, we identified the ion transporter NKCC1 as a suppressor of LAT1 and mTORC1 activation. We are now investigating the mechanisms by which ions regulate signal transduction pathways that control mTORC1 activation and cellular growth.
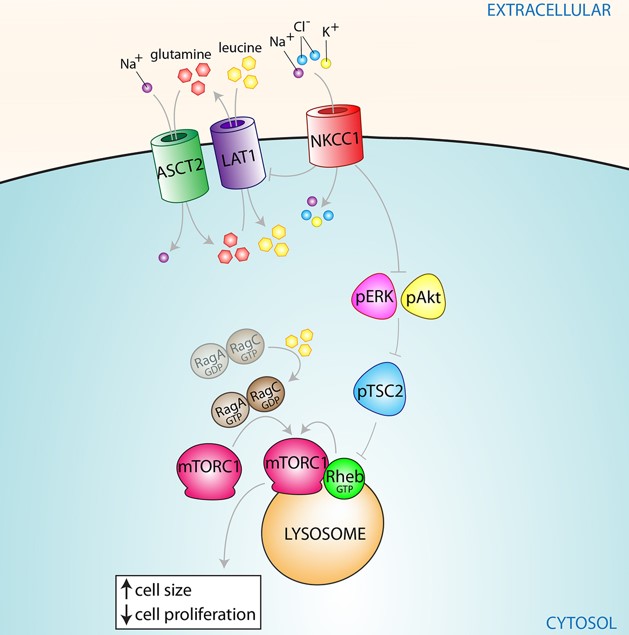
NKCC1 inhibits the mTORC1 pathway.
Description of image: The ion transporter Na+/K+/2Cl- inhibits the activity of the leucine transporter LAT1 and the Erk and Akt signaling pathways; These proteins and signaling pathways are known to activate the master regulator mTORC1. mTORC1, translocated to the lysosomal membrane by elevated leucine levels in the cell, is responsible for regulating protein synthesis, cell proliferation and cell size, as well as inhibiting autophagy.
Cystic Fibrosis (CF)
We have been working on two aspects of CF research:
- the relationship between ENaC and CF, where we showed that ablation of Nedd4L in mouse lung epithelia causes CF-like lung disease due to excessive ENaC expression/function, mimicking the excess ENaC function upon loss of CFTR in CF lungs;
- searching for kinase inhibitors that promote rescue of mutant CFTR (F508del), the major mutation in CF.
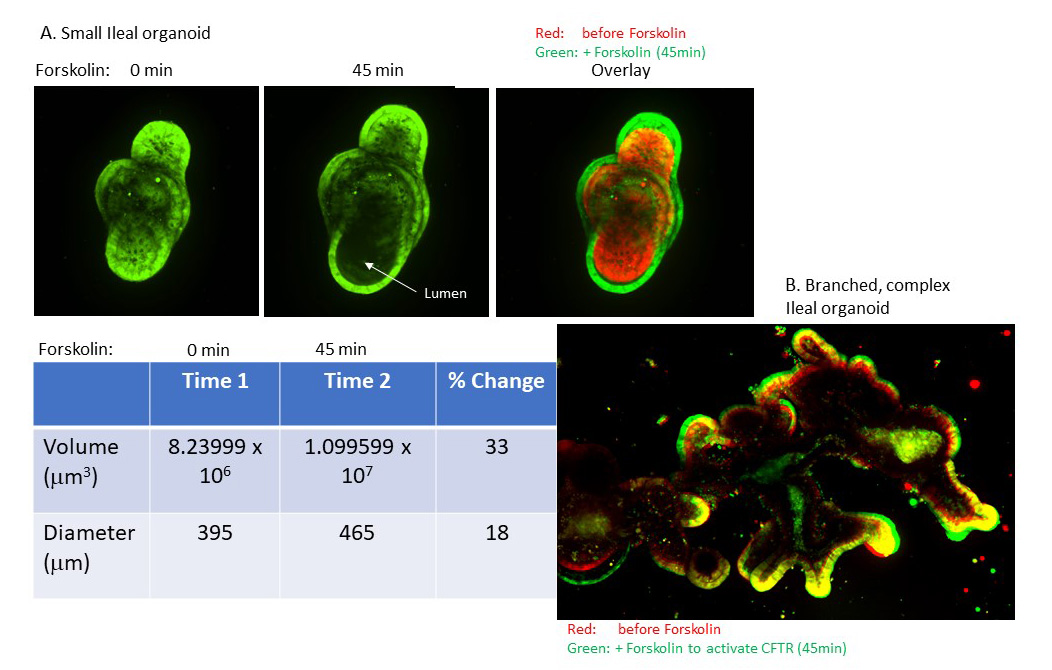
Activation of CFTR in intestinal organoids.
Description of image: The Cystic Fibrosis Transmembrane conductance Regulator (CFTR), which is mutated in cystic fibrosis, is expressed in ileal mouse tissue organoids and can be activated with forskolin. Activation is detected by increased size/volume of the organoid, and is quantified by IXMC, a specialized high content confocal microscope that enables phenotypic and drug screens in 3 dimesion structures, like organoids.
Earlier work
Earlier work in the lab also focused on receptor Tyrosine phosphatases, especially PTPsigma, but these studies are no longer carried out in the lab.
