Collageneous lectins are innate immune pattern recognition opsonins. They recognize microbial pathogens, NETs and dying cells. We aim to identify how collectins regulate infection and inflammation, and to improve the quality of specific collectins such as surfactant protein D (SP-D) for therapeutic use.
Antibodies of the Innate Immune System
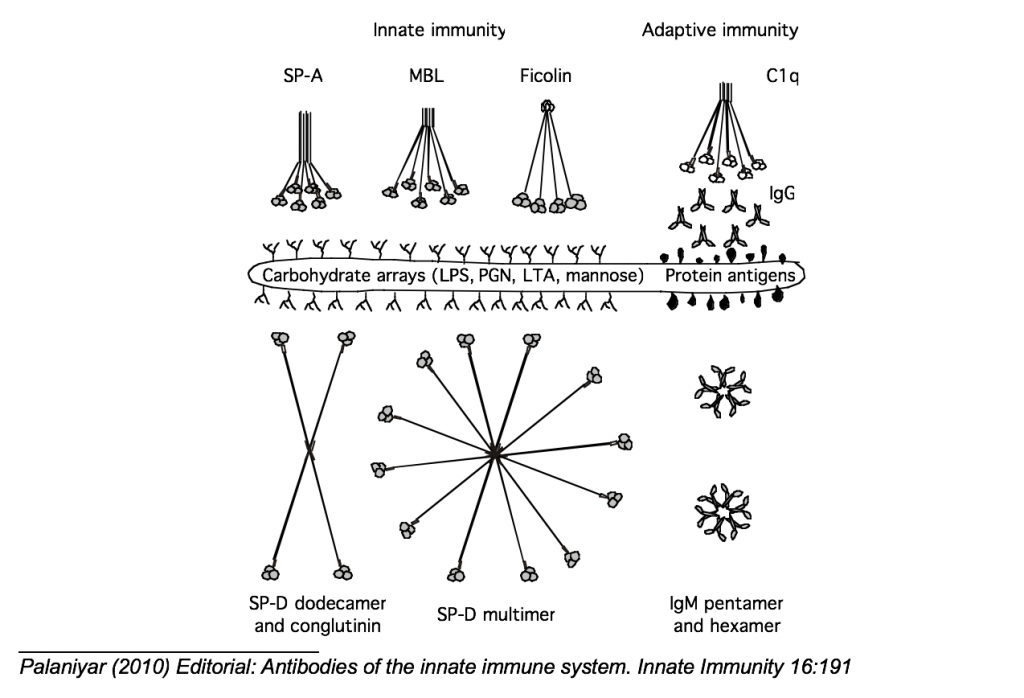
Collectins (Collagenous Lectins) come in many forms, from receptors to soluble innate immune proteins. Collectins compose a large portion of the proteins involved in the innate immune response; SP-D, SP-A, MBL, Ficolin, all contain a collagen-like domain and recognize carbohydrates. This carbohydrate-recognizing ability allow these molecules to recognize sugars found on bacteria, fungi, and other pathogens.
SP-D binds DNA
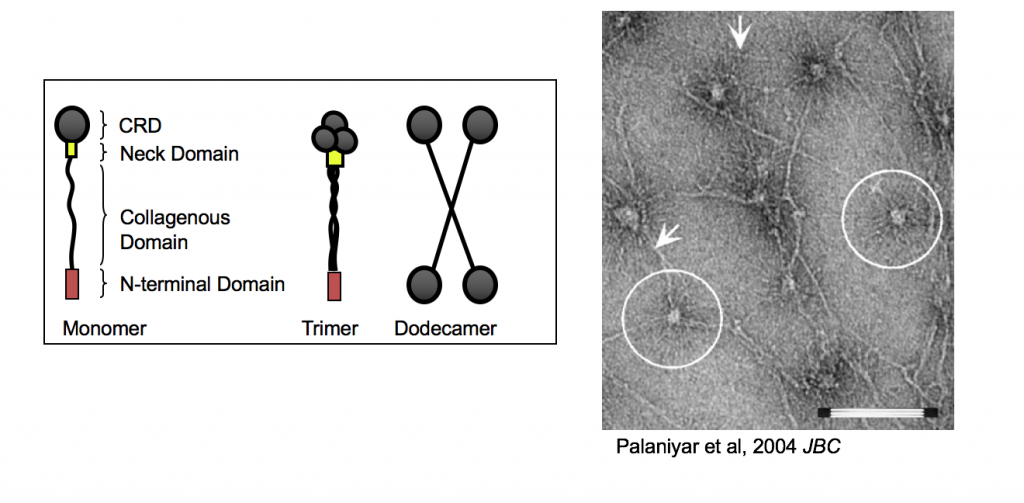
Surfactant Protein D (SP-D) can come in many forms from monomers to dodecamers to multimers (Asterisk-shape), although their most basic form is composed of a Carbohydrate-Recognizing Domain (CRD) that is connected to a peptide strand composing of a collagenous domain via a neck domain. The figure on the right showcase the SP-D (Circles) preferentially binding to DNA strands (Arrows) via their CRD and collagen-like regions.
SP-D agglutinates and brings bacteria to NETs
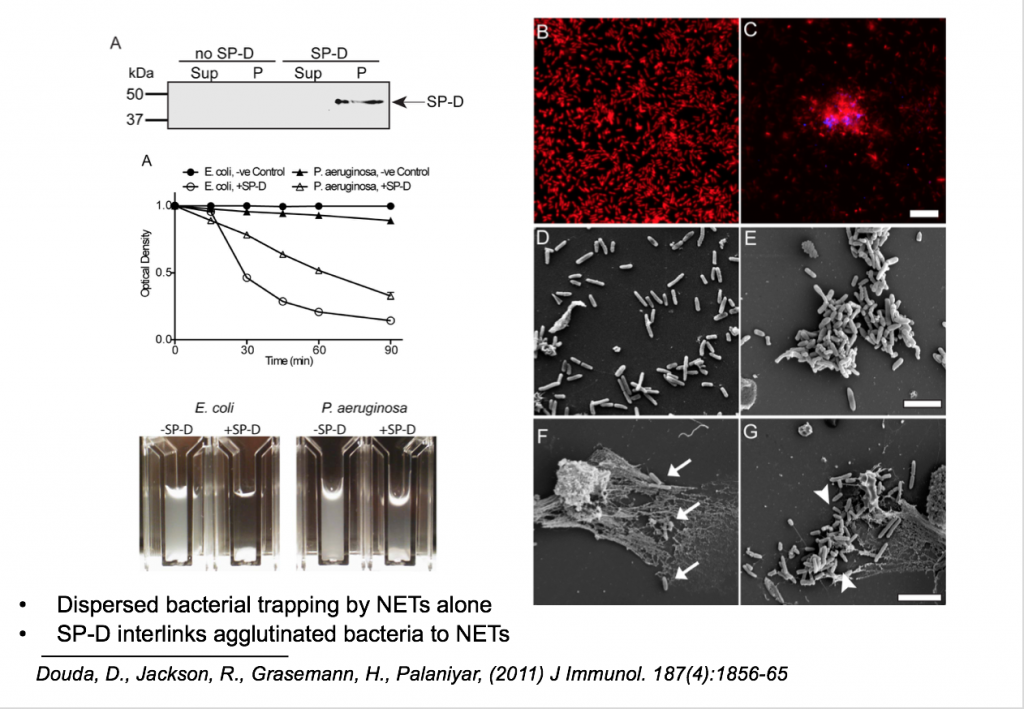
SP-D was found to bind to bacteria such as E. coli and P. aeruginosa as shown on the left, where the presence of SP-D made the bacteria-infected solution more clear compared to the solution containing only bacteria. This phenomenon was replicated under the microscope, where P. aeruginosa (Red) were found to be agglutinated in the presence of SP-D (Blue). This phenomenon was replicated again under the electron microscope, where you can see rod-shaped bacteria originally dispersed in (D) become agglutinated in the presence of SP-D in (E).
Interestingly, SP-D enhances the number of bacteria trapped by NETs in (G) compared to (F).
A model for SP-D-mediated increase in NET function and clearance
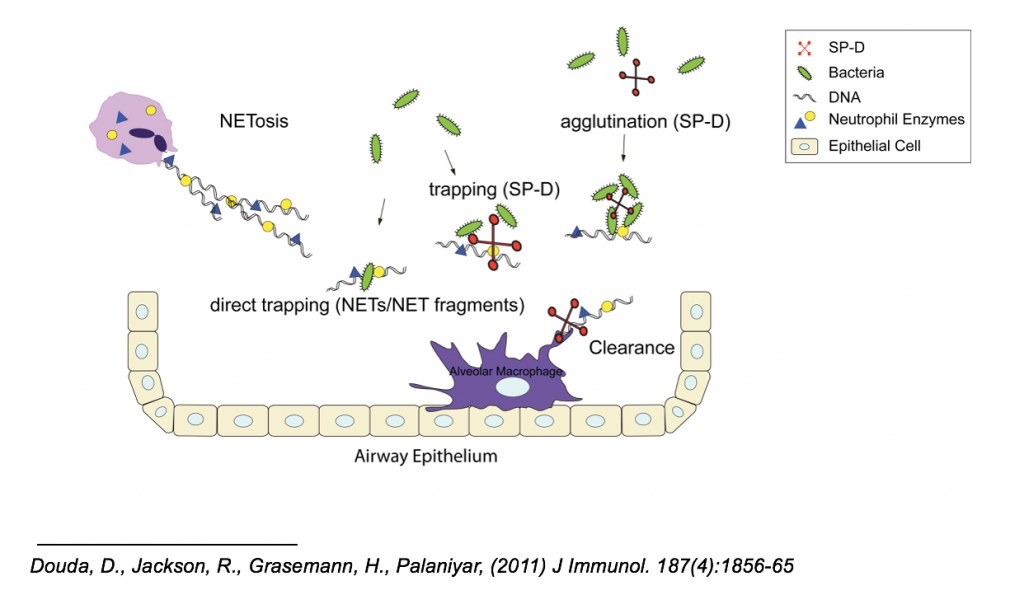
SP-D has the ability to bind to DNA, and the ability to bind to carbohydrates. These two abilities allow SP-D to greatly aid in NET functions, as well as aid in the clearance of NETs after the infection is cleared. SP-D can aid in NET functions by agglutinating or concentrating bacteria by binding to multiple carbohydrates on these bacteria such that these masses of pathogens become easier for NETs to trap them. SP-D can also while binding to bacteria, also bind to the DNA strands of NETs, facilitating trapping of the pathogens. After the infection is resolved, SP-D can bind to the fragments of NETs via its DNA-binding ability, and promote macrophage uptake of these fragments for clearance.
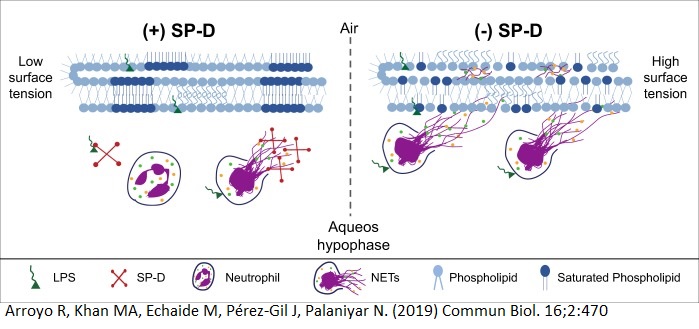
A model for SP-D suppressing LPS-mediated NETosis and NET-mediated inhibition of surfactant
After further research into SP-D in the context of bacteria infection of the lungs, we found that SP-D play more roles than just enhancing NET function and clearance; SP-D can also suppress LPS (Lipopolysaccharide)-mediated NET formation and prevent NET-mediated inhibition of the surfactant layer in the lungs. SP-D can act as a competitive inhibitor of NET formation by competing with neutrophils to bind to LPS, a carbohydrate found on gram-negative bacteria that is also a common activator of NET formation. In terms of the surfactant layer, they are necessary to reduce surface tension in the lungs, but excess NETs within the lungs will disturb the structure of the surfactant layer and thus their function of reducing surface tension. If surface tension within the lungs are too great, it can lead to the collapse of the alveoli, the air-breathing structures of the lungs.