Once released by hydrolases in the lumen of (endo)lysosomes, the smallest components of organic cargo are transported across the membrane and into the cytosol. This is achieved by the solute carrier (SLC) group of membrane transport proteins (which include over 400 members) that are categorized into 66 families, according to the solutes they are responsible for transporting. While many of these transporters are found on the surface of cells, we are largely interested in the members of the SLC family that are located to the endolysosomal pathway. This is because 1) these transporters are poorly understood when compared to their counterparts that reside in the plasma membrane, 2) defects in these transporters can lead to lysosomal storage disorders, and 3) variants in these genes are also associated with inflammatory and neurodegenerative diseases. We are studying the mechanisms that provide the driving force and activity of select members of SLC transporters. We are also interested how these transporters regulate (and are regulated by) the physical properties of the membranes in which they reside including its tension and electrical potential.
Phagocytosis, the ingestion of particulate matter, plays an essential role in the maintenance of tissue homeostasis. It serves as a first line of defense in the elimination of invading pathogens. Phagocytosis also prevents secondary necrosis and unwanted inflammation by efficiently recognizing and disposing of apoptotic bodies and debris. In addition, phagocytic clearance of malignant cells is fundamental in the innate immune surveillance for cancerous growth; indeed, suppression of phagocytosis facilitates tumor-mediated immune evasion. Given these essential functions, phagocytes reside in virtually all tissues of the body, where they constantly survey their surroundings for prey.
In their surveillance, phagocytes must rapidly distinguish harmful from healthy components by detecting features exposed on the surface of their putative targets. Features that trigger phagocytosis can be intrinsic to the target or facilitated by the deposition of soluble opsonins on the target. These ligands are called “eat me” signals as they engage phagocytic receptors that trigger extensive remodeling of the plasma membrane and of the actin cytoskeleton, culminating in the extension of pseudopods that surround and engulf the target.
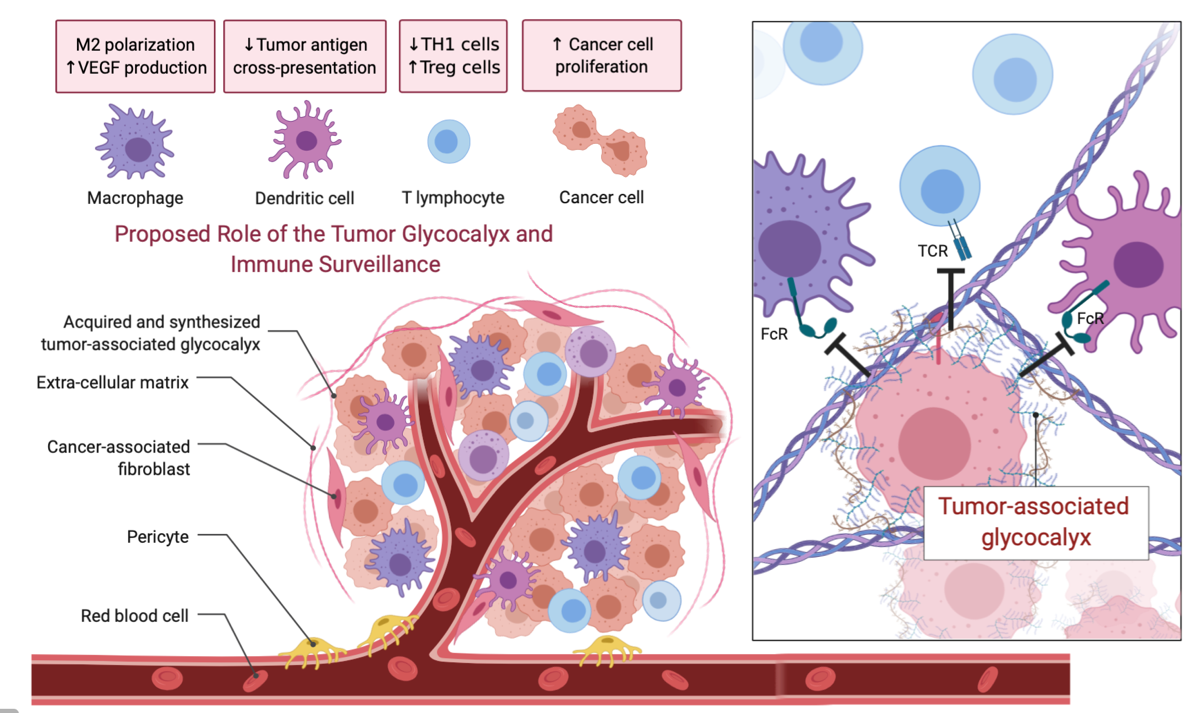
In addition to scanning for “eat me” signals, phagocytes also recognize surface molecules that serve as “don’t eat me” signals. These include CD47, PD-L1, and CD24 that engage their cognate receptors SIRPα, PD-1, and Siglec-10, respectively, to exert an inhibitory effect on phagocytosis. When engaged, these inhibitory receptors arrest signaling pathways by recruiting otherwise cytosolic phosphatases that suppress phagocytic signaling. Certain tumors have found ways to usurp these mechanisms to support their growth. In addition, mucinous tumors form an elaborate barrier to the recognition of “eat me” signals and neo-antigens: the glycocalyx. This can be considered a “don’t come close to me” barrier to phagocytosis and cytolytic interactions with killer cells.
While IgG-based biologics that target the “don’t eat me” pathways of cancerous lesions have been deployed with some success, augmenting these responses, for example by removing the glycocalyx barrier, would be advantageous.
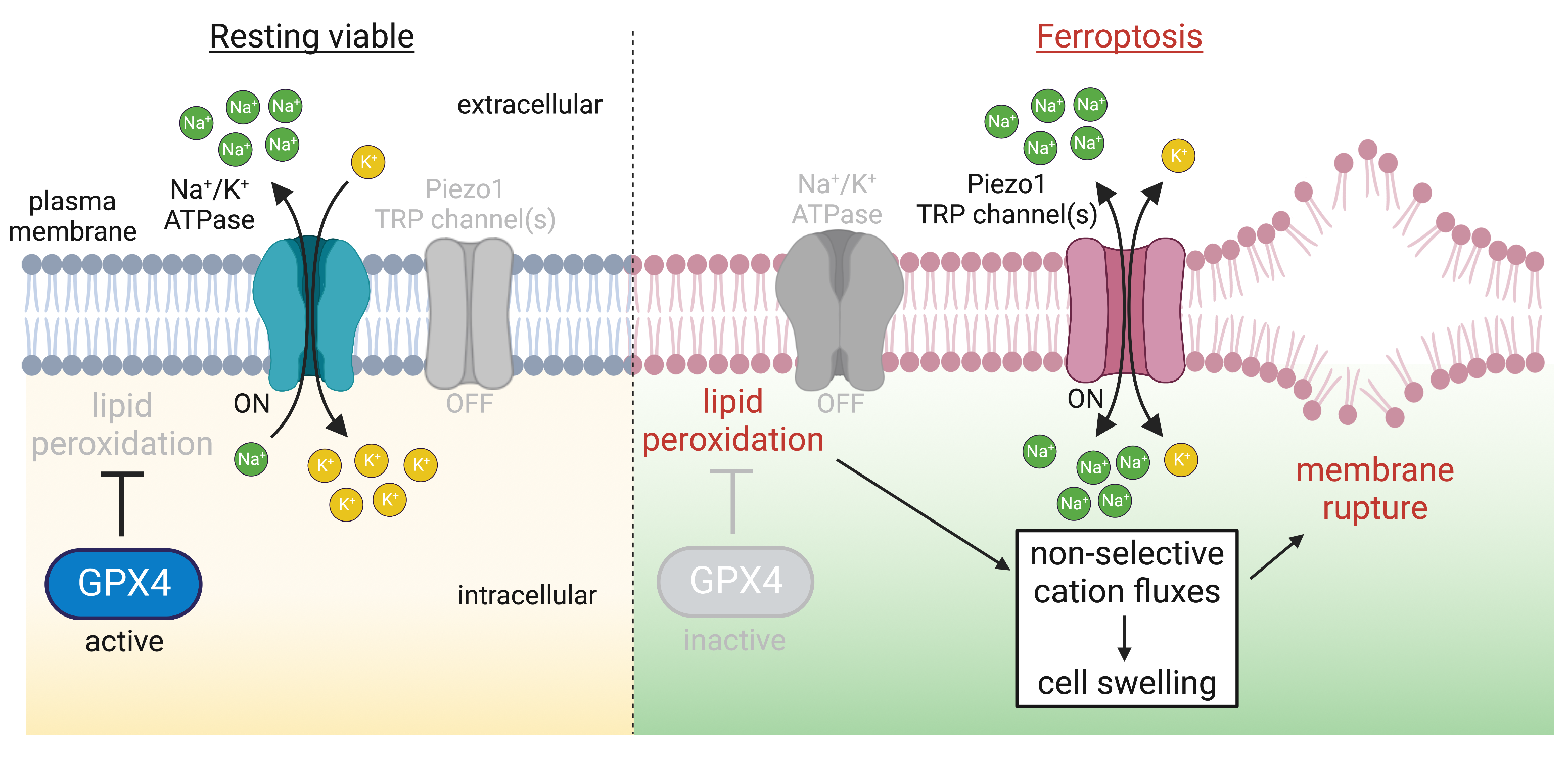
The ongoing metabolic and microbicidal pathways that support and protect cellular life generate potentially damaging reactive oxygen species. To counteract damage, cells express peroxidases, antioxidant enzymes that catalyze the reduction of oxidized biomolecules. A single hydroperoxidase is responsible for reducing lipid peroxides called Glutathione peroxidase 4 (GPX4); its activity is essential and its inhibition causes a unique type of lytic cell death, ferroptosis.
Recently, we have been working to understand the mechanisms that lead to cell lysis in ferroptosis. Using sensors for membrane oxidation that we can image with high resolution microscopy, we found that the lipid peroxides formed during ferroptosis accumulate preferentially at the plasma membrane. The oxidation of the membrane increases in its tension, resulting in the constitutive gating mechanosensitive channels, ultimately making the membrane permeable to cations. Blocking or deleting the channels prevents ferroptosis.
We’re now interested in how oxidized membranes feature more generally in mechanosensitive channel gating, including for organelles.