OUR MISSION
We aim to discover basic mechanisms that control gene expression and epigenetic reprogramming. We strive to apply this knowledge through manipulation of induced pluripotent stem (iPS) cells to model human disease and test potential therapies for personalized medicine.
a
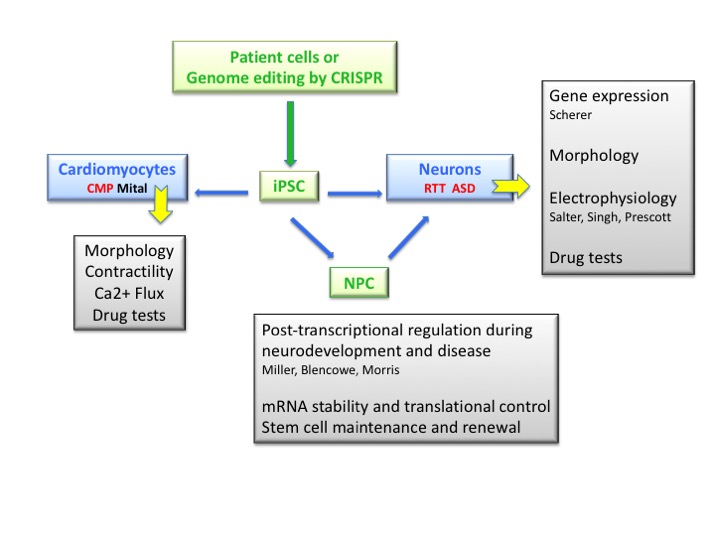
Ongoing research in our group centers on disease modeling and drug screening using induced Pluripotent Stem (iPS) cells. Initially we used a novel EOS reporter vector to reprogram patient fibroblasts into iPS cells and more recently we perform CRISPR gene editing to create isogenic cell pairs. Next, Neural Progenitor Cells (NPC) are generated to produce neurons for defining phenotypes and expression patterns that are related to Rett syndrome (RTT) and autism spectrum disorder (ASD).
We also collaborate with the Mital lab to evaluate the cardiomyocyte phenotype of pediatric cardiomyopathies. Drug testing is then performed to rescue the patient-specific in vitro phenotypes. Overall, our goal is to utilize reprogramming technology to repurpose or identify novel drugs for personalized medicine.
Recent Publications
- Abnormal Lipid Signaling Characterizes Diastolic Dysfunction in Pediatric Cardiomyopathy. Turinsky AL. et al., 2026. JACC: Basic to Translational Science, 11(3):101491
- Diversifying the iPSC reference line concept. Ellis J. et al., 2025. Cell Stem Cell, 32(6):873-877
- Hypersynchronous iPSC-derived SHANK2 neuronal networks are rescued by mGluR5 agonism. McCready FP. et al., 2025. Stem Cell Reports, 102718
- Myosin inhibitor reverses hypertrophic cardiomyopathy in genotypically diverse pediatric iPSC-cardiomyocytes to mirror variant correction. Kinnear C. et al., 2024. Cell Reports Medicine, 5: 101520
- Buffering of transcription rate by mRNA half-life is a conserved feature of Rett syndrome models. Rodrigues D.C. et al., 2023. Nature Communications, 14:1896
The Ellis Lab is not hiring in 2026.
a