Dendritic spines
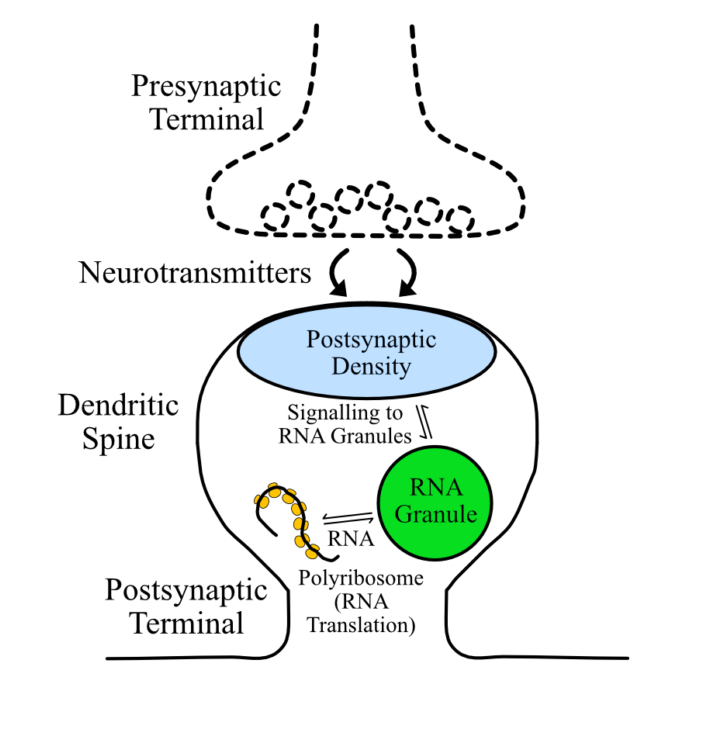
Communication between neurons in the brain requires exquisitely controlled, precise connections between neighbouring cells. The connections, or synapses, are composed of a presynaptic bouton at the end of axons and a postsynaptic dendritic spine that protrude from the surface of dendrites.
Within the dendritic spine are different condensates that can form through the process of biological phase separation, including postsynaptic densities. This condensate controls dendritic spine plasticity that is required for basic brain functions including memory, behavior, and emotions.
Mutations that have been identified in many proteins that localize to the postsynaptic density are linked to neurodevelopmental disorders such as autism spectrum disorder, schizophrenia, and intellectual disabilities. Moreover, the biochemical and biophysical mechanisms by which these mutations alter normal dendritic spine function are unknown.
We are particularly interested in understanding the role of phase separation in regulating postsynaptic density controlled local RNA translation and actin polymerization; two processes that regulate synaptic plasticity and communication between neurons.
T cell signaling: immune synapse organization
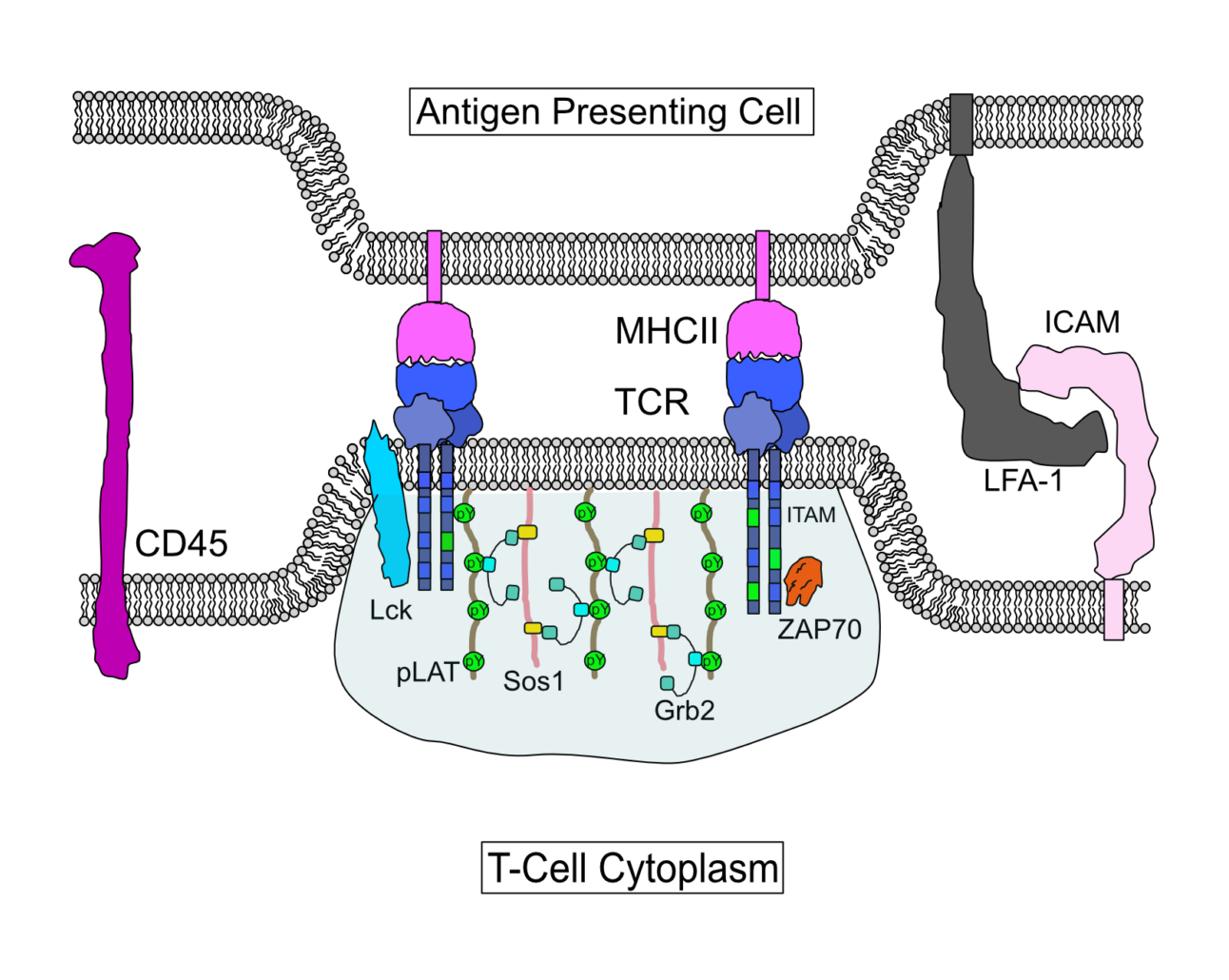
T cell response to infection relies on the recognition of peptide major histocompatibility complexes (pMHC) presented on the surface of antigen presenting cells by T cell receptors on the surface of T cells.
Upon T cell receptor binding to pMHC, kinases are activated within the T cell and signaling clusters composed of proteins form on the membrane. Binding of T cell receptor to pMHC also alters the organization of lipid domains on the T cell membrane.
It is unclear whether lipid and protein domain organization is coupled, and whether potential coupling is required for T cell activation. We are interested in understanding organization at the membrane of the immune synapse and the role that co-existing lipid and protein domains play in regulating T cell activation.
Heterogeneous condensation
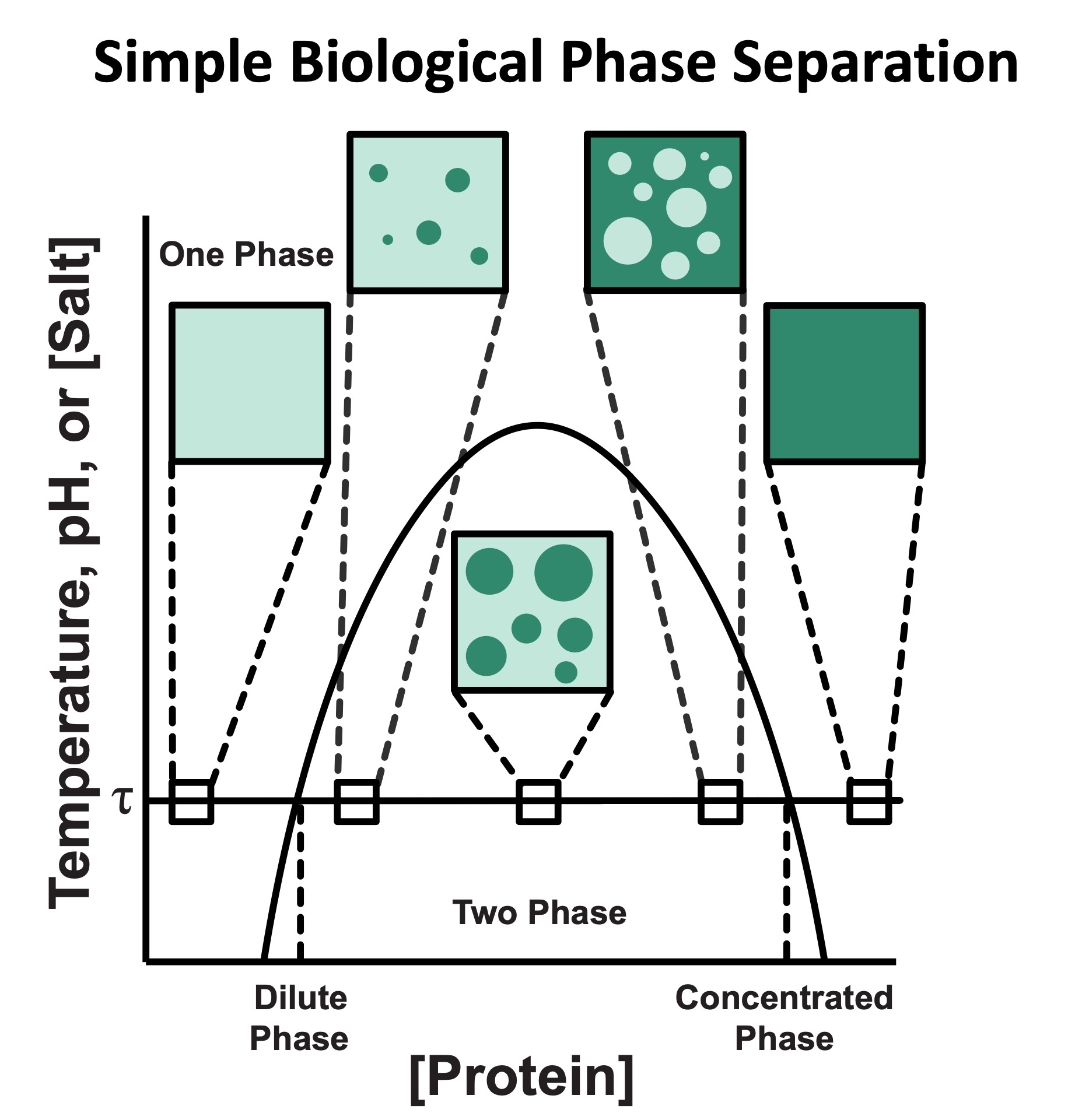
Many biomolecular condensates on membranes, in the cytosol, and in the nucleus form through the process of biological phase separation.
Initial biophysical analysis of biological phase separation was often performed on single component systems where a single protein interacted with itself and underwent phase separation in specific buffer conditions. In these simple systems, the concentrations of protein inside and outside of the condensates remain constant while the volume of condensed material increases when additional protein is added to the solution.
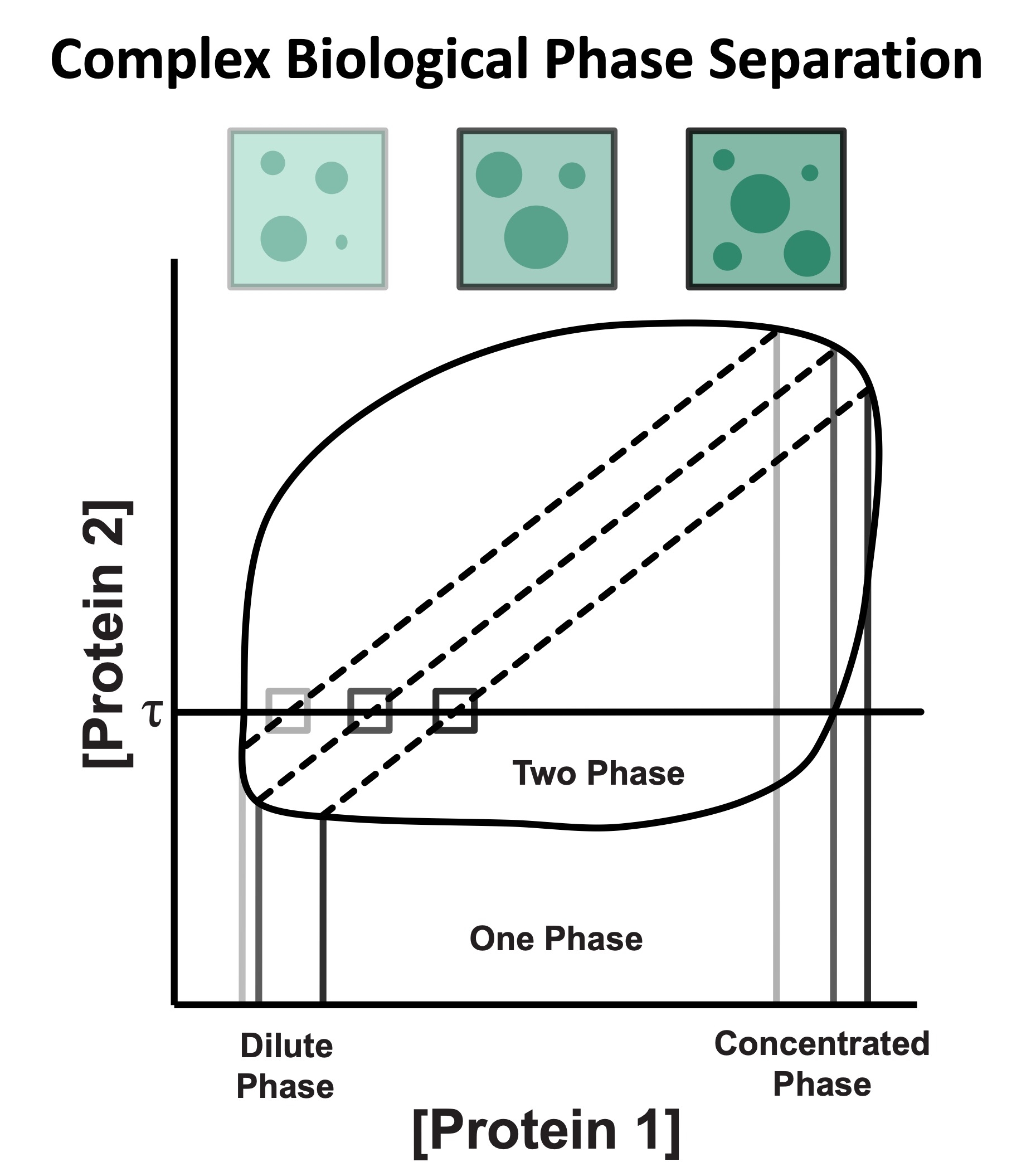
Many condensates are complex structures whose existence relies on interactions between multiple binding partners. In these more complex systems, it is not clear how the concentrations of proteins inside and outside the condensate will change as the total concentration of proteins is increased in solution. Furthermore, the role that each component plays in promoting biological phase separation is unclear.
Using model systems, we are interested in understanding a potential buffering role for complex condensates and deciphering general principles that underlie the contribution of different components to multi-component condensates.