The Youn lab utilizes functional proteomics to determine molecular mechanisms underlying neurological disorders and develop novel proteomics screening strategies to identify therapeutic candidates.
Stress granule proteins and their interactome
Team Members: Kiki Huang
We are interested in understanding the networks of protein-protein and protein-RNA interactions that underlie stress granule formation and dissolution.
We utilize a proximity-dependent biotinylation technique, called BioID, to probe many different subdomains of stress granules and obtain a comprehensive view of the organelle architecture.
Stress granules are enriched for intrinsically disordered proteins with unclear functions. We focus on elucidating the role of these intrinsically disordered proteins.
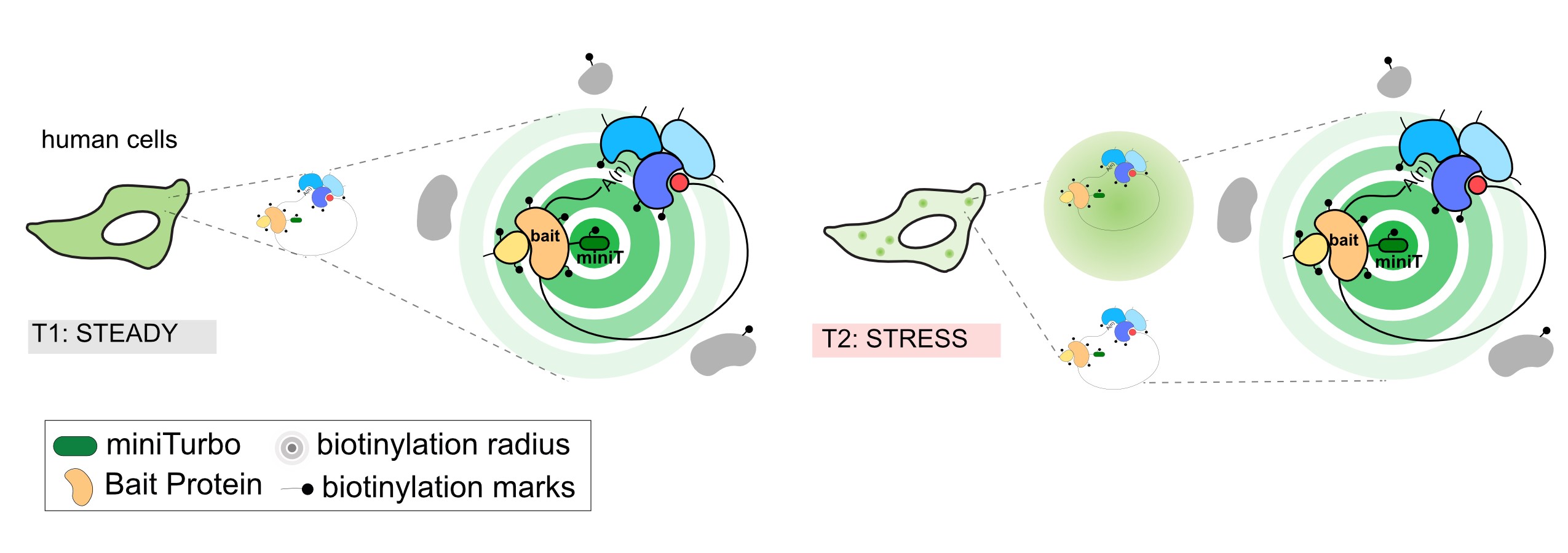
Diagram of proximity-dependent biotinylation in living cells
Uncovering the role of stress granules in post-transcriptional regulation
Team Members: Eileigh Kadijk and Alan Minkovich
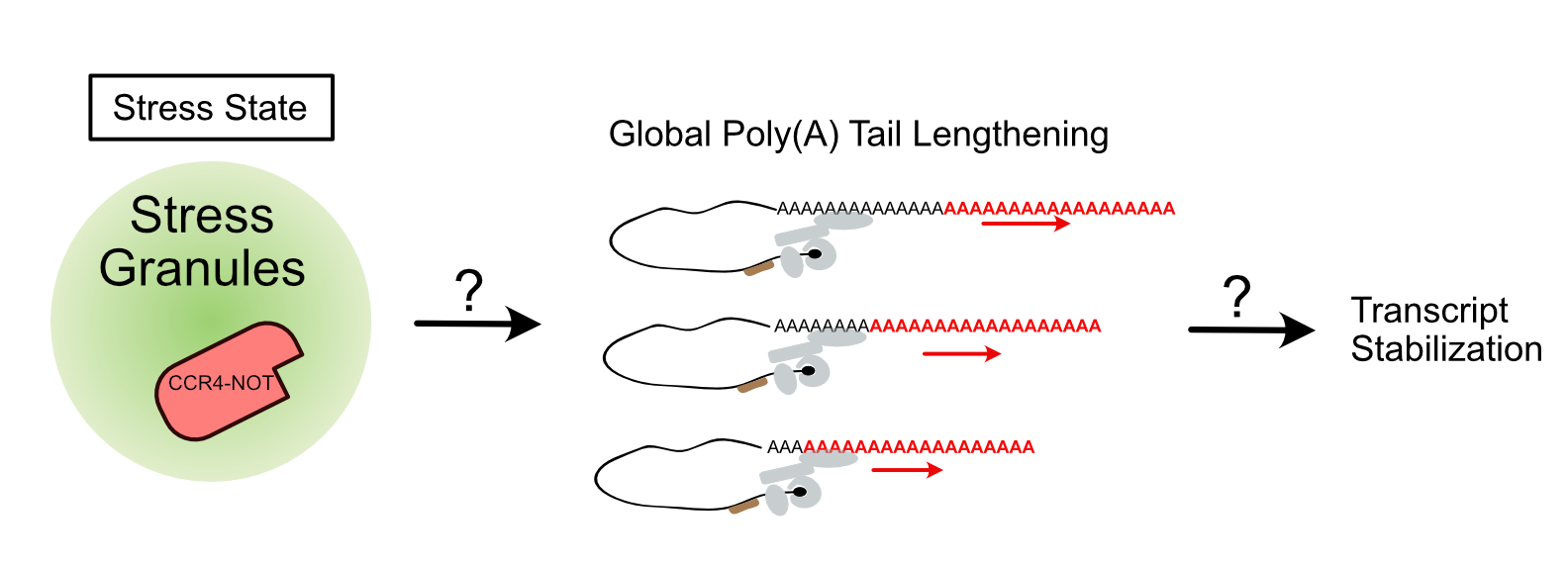
Diagram showing the relationship between stress granules and post-transcriptional regulation.
The exact purpose for stress granules within cells has been heavily debated. Its principle components, RNA and RNA-binding proteins, and their appearance during translational stalling, has implied a role for these condensates in post-transcriptional regulation. Our lab is interested in the role of stress granules in sequestering the master regulator of mRNA decay, CCR4-NOT deadenylase complex during stress conditions. CCR4-NOT shortens poly(A) tails of mRNA, affecting RNA stability and translation. To examine the role of stress granules in regulating CCR4-NOT activity, we use discovery and targeted approaches to detect changes in transcript poly(A) tails and their abundance under stress.
Elucidating the role of TDP-43 and its dysregulation in neurodegenerative disease
Team Members: Karl Schreiber and Carina Lyons
The neurodegenerative disease amyotrophic lateral sclerosis (ALS) involves the deterioration and death of motor neurons, causing a progressive loss of motor functions that is ultimately fatal. In the majority of ALS patients, aggregates of a protein called TDP-43 are found in motor neurons. Aberrant phase transition and aggregation of TDP-43 cause ALS pathogenesis (left). In healthy state, this protein is capable of binding both RNA and DNA and appears to influence multiple cellular processes. However, we lack a complete understanding of the role of TDP-43 in normal physiological activity as well as its contribution to ALS pathogenesis. We use proteomic approaches to identify the proteins associated with TDP-43 in various subcellular compartments (right) and to monitor how this molecular neighbourhood changes in the presence of different TDP-43 mutants or in response to cellular stress. These insights can help us narrow in on key aspects of TDP-43 activity as potential therapeutic targets.
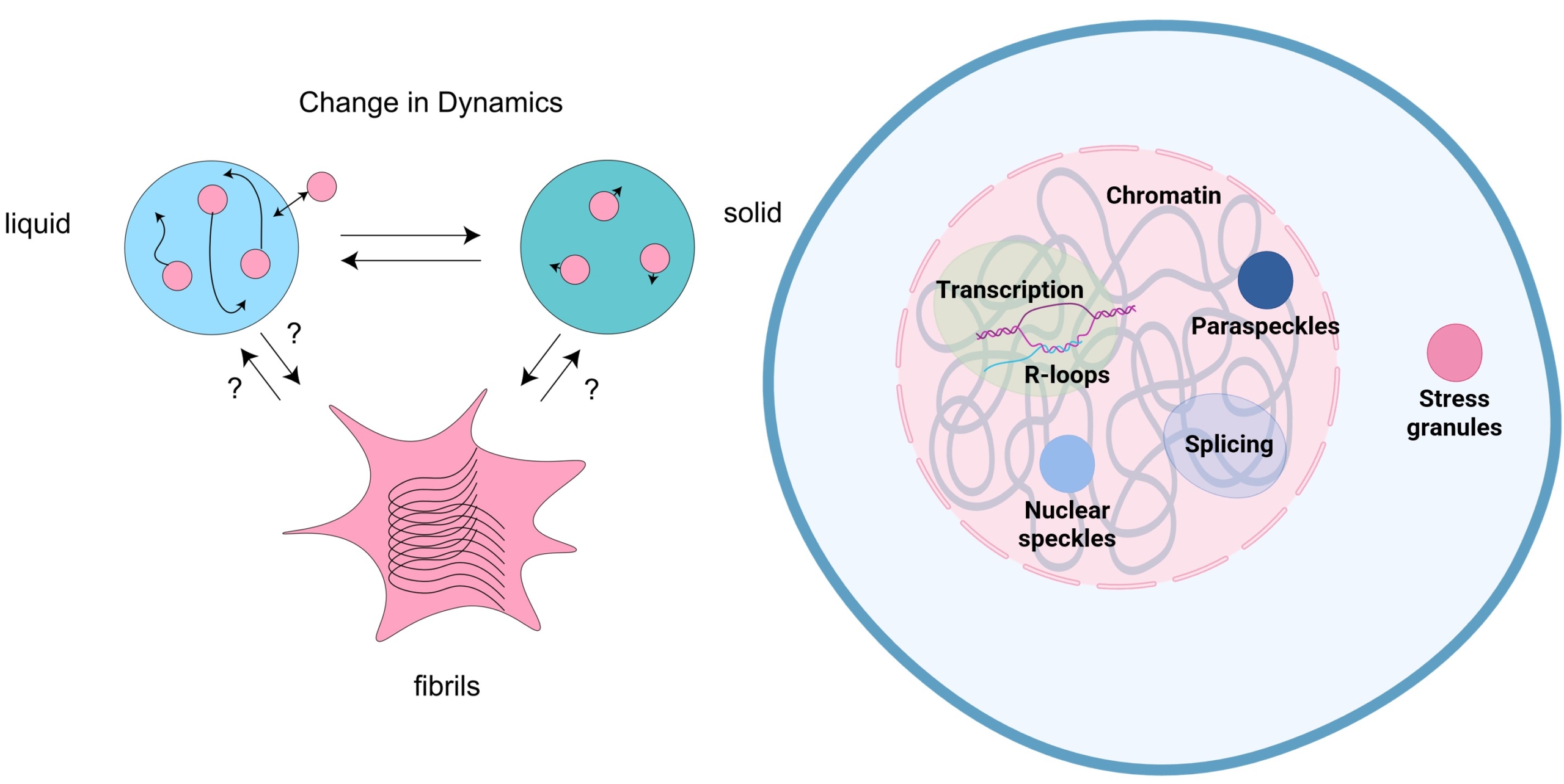
Left: TDP-43 undergoes phase transition from liquid to solid and fibril-like aggregates in vitro.
Right: Subcellular compartments and biological processes in which TDP-43 associates with.
Stress granules and amyotrophic lateral sclerosis
Team Members: Rachel Chiang
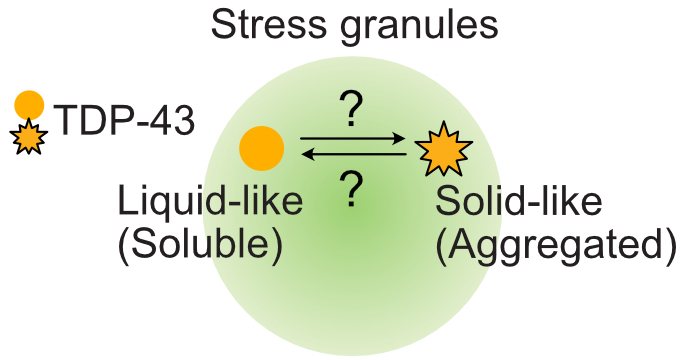
The role of stress granules in TDP-43 aggregation remains controversial
We are interested in investigating the contribution of stress granules to the pathogenesis of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). In patient samples, the essential nuclear DNA/RNA binding protein TDP-43 mislocalizes to the cytoplasm. Its nuclear depletion and subsequent aggregation result in both gain of toxicity and loss of function. Notably, TDP-43’s localization to stress granules has been observed under stress, but the contribution of these biomolecular condensates to ALS pathology remains unclear. Therefore, we aim to determine if stress granules promote or prevent TDP-43’s cytoplasmic aggregation to better understand disease progression.
Modulating RNA-rich biomolecular condensates using synthetic minibinders in collaboration with Dr. David Baker
Team Members: Faizah Abdullah
Miniprotein binders, or minibinders, are computationally-designed proteins of ~100 amino acids which are able to bind to specific regions of a target molecule with high specificity. Dr. David Baker and his team at the University of Washington have recently showed that proteins containing intrinsically disordered regions can be targeted using these minibinders. In collaboration with the Baker Lab, we are investigating the use of these minibinders in modulating the phase separation ability of RNA-rich biomolecular condensates with the aim of developing therapeutics to treat diseases displaying dysfunctional phase separation capacity, such as amyotrophic lateral sclerosis (ALS).
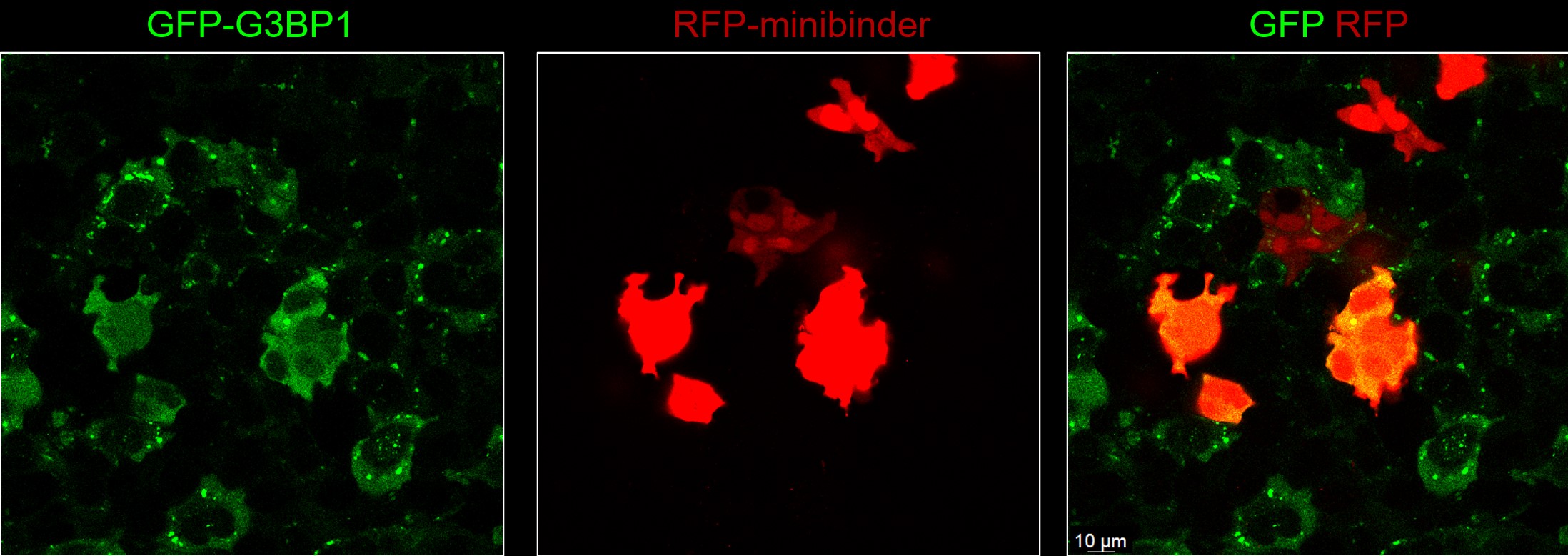
HeLa cells co-expressing GFP-tagged G3BP1 and RFP-tagged G3BP1 minibinder, G3BP1-11, under oxidative stress. G3BP1-11 minibinder-expressing cells show diminished stress granules. (G3BP1-11 from Liu, C and Baker, D et al., Nature 2025)
