Youn Lab
Unraveling the Proteomes of Biomolecular Condensates Important for Disease
The Youn lab is located at The Hospital for Sick Children’s Research Institute. Headed by Dr. Ji-Young Youn, the lab studies the proteomics of biomolecular condensates important for cellular adaptation to stress and disease. Biomolecular condensates are dynamic, membrane-less cellular compartments formed by phase separation of specific biomolecules—typically proteins and nucleic acids—that concentrate and organize biomolecules without enclosing them in lipid bilayers. We utilize combination of proteomics, microscopy, genetic and synthetic tools to study RNA-rich biomolecular condensates important for cellular adaptation to stress and disease.
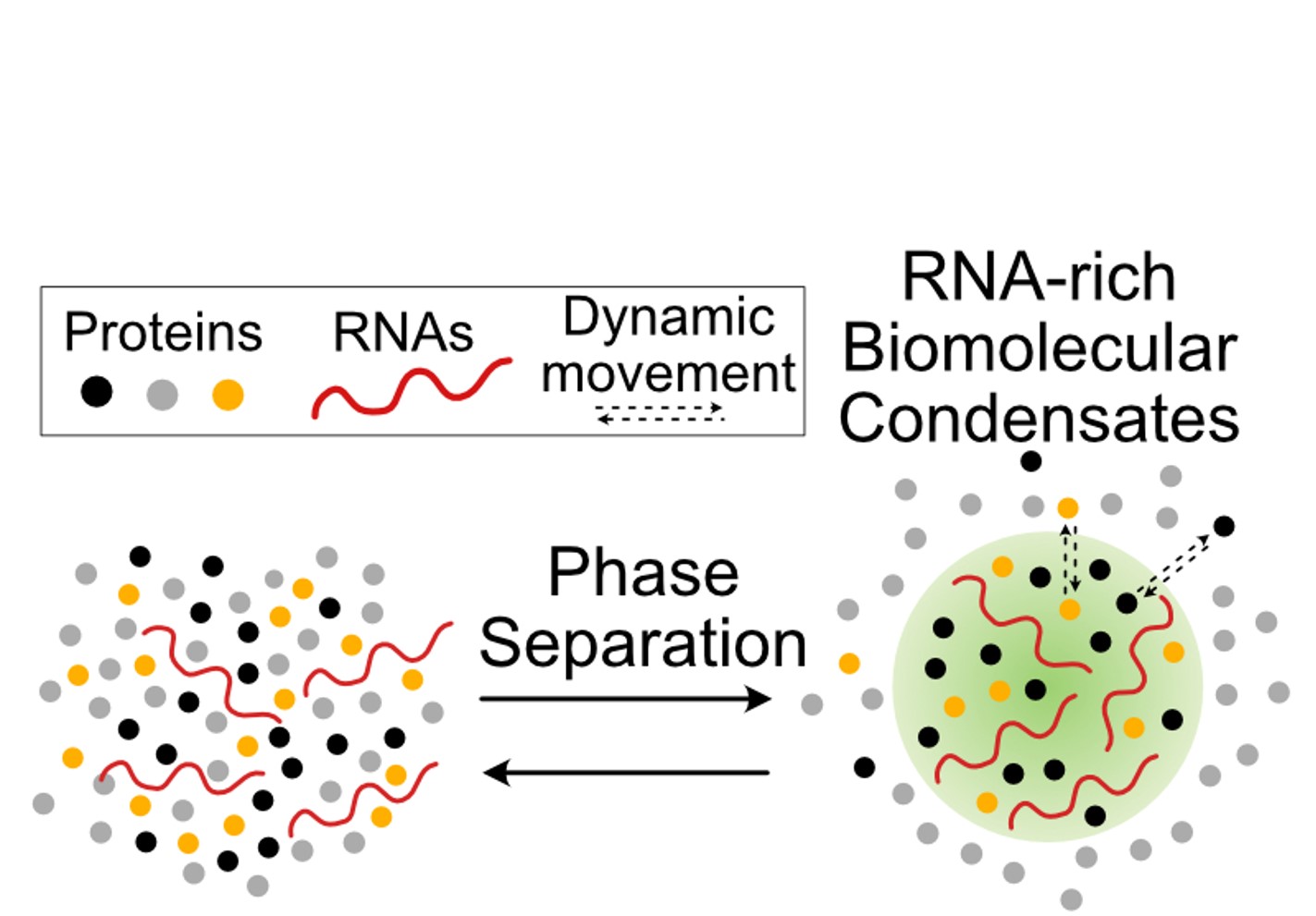
RNA-Rich biomolecular condensates are formed by phase separation of RNAs and proteins, creating a unique microenvironment.
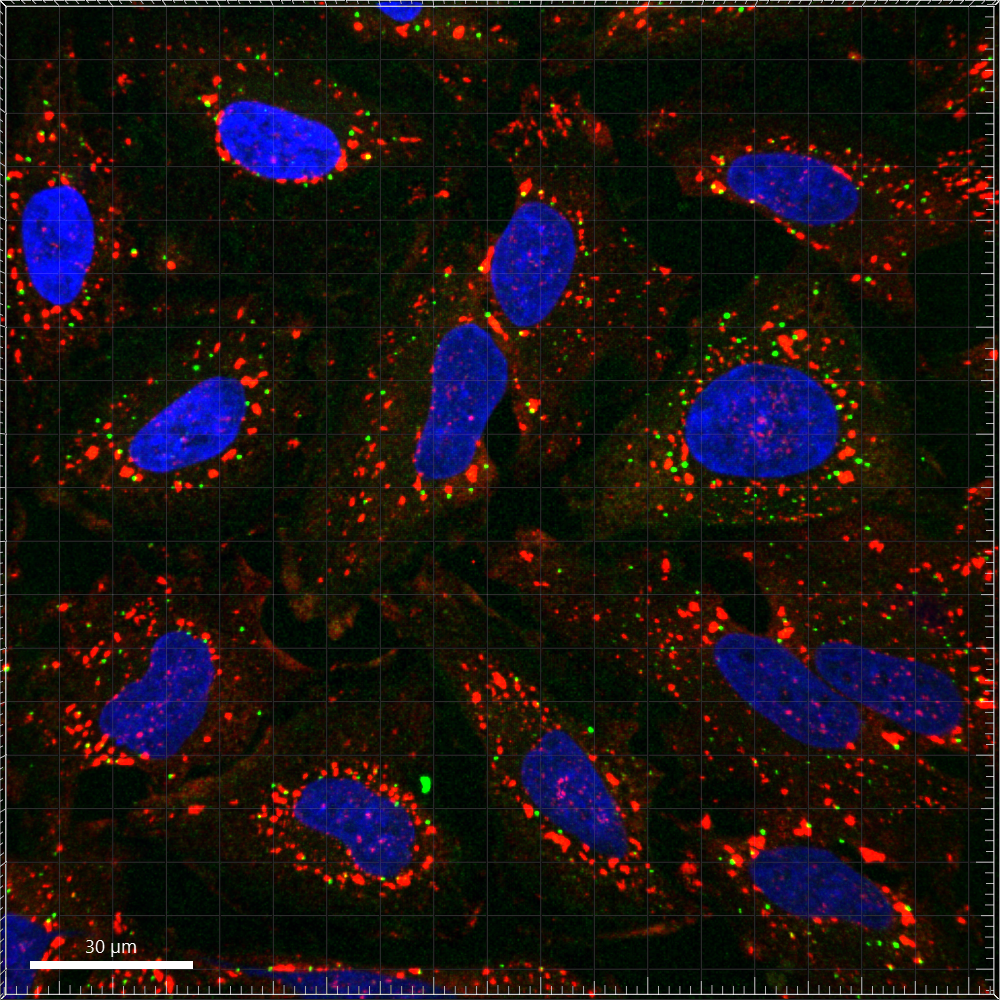
Immunofluorescence image of human HeLa cells under oxidative stress, stained with a stress stained with granule marker (G3BP1, Red) and a processing-body marker (EDC3, Green) and DAPI (nucleus).
Our Focus
Stress Granule
Living organisms are constantly challenged by stressful conditions. To cope, their cells have evolved to utilize specialized biomolecular condensates that rapidly respond to cellular needs. These include stress granules, which are found in all eukaryotic organisms. Stress granules form in the cytoplasm when the protein production halts upon stress; proteins and RNAs involved in protein production partition themselves into discrete droplets.
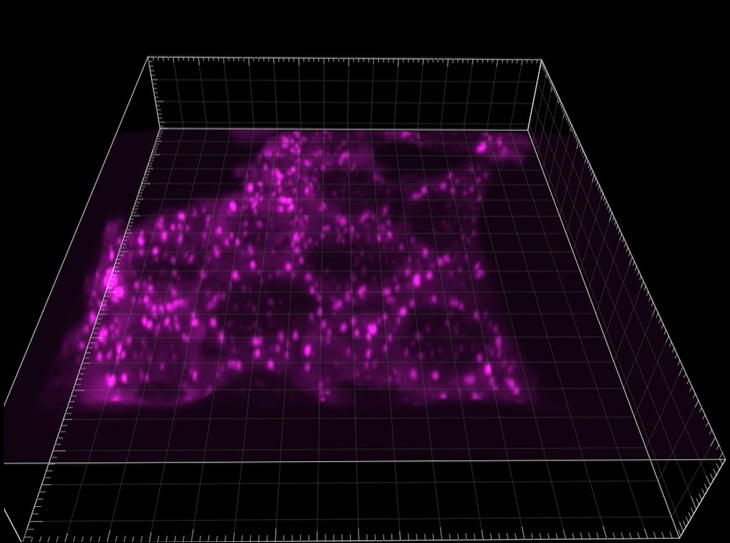
Fluorescence image shows stress granules formed in HAP1 cells. Stress granule marker, G3BP1, is endogenously tagged with mScarlet.
Neurodegenerative Diseases
We investigate how dysregulated biomolecular condensates contribute to neurodegenerative diseases.
We currently focus on TDP-43 (TARDBP) and FUS proteins, whose loss and gain-of-function cause amyotrophic lateral sclerosis disease. Disease-causing mutations affect their phase separate propensity and/or localization, impacting on their module functions.
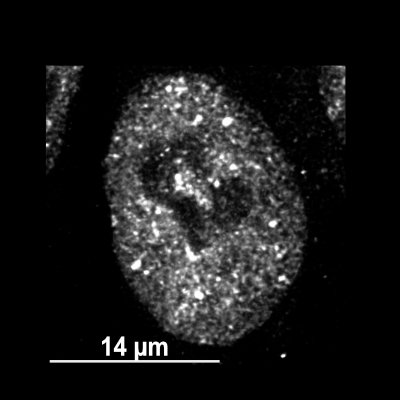
Fluorescence image shows stress granules formed in HAP1 cells. TDP-43 (TARDBP) is endogenously tagged with mScarlet.
Neurodevelopmental Disease
Post-transcriptional regulation is crucial for neurodevelopment. Proteins involved in post-transcriptional regulation are often enriched in RNA condensates.
Genetics show a striking enrichment of neurodevelopmental disease-causing or associated mutations in genes encoding for RNA condensate proteins, implicating dysregulated RNA condensates in neurodevelopmental diseases.
We investigate how neurodevelopmental disease causing mutations affect the localization and function of the encoded protein.
