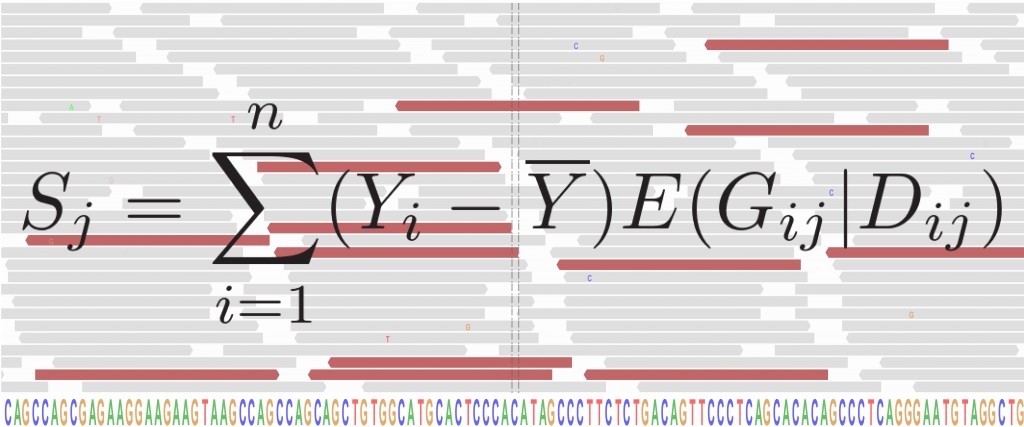Genetic modification in cystic fibrosis
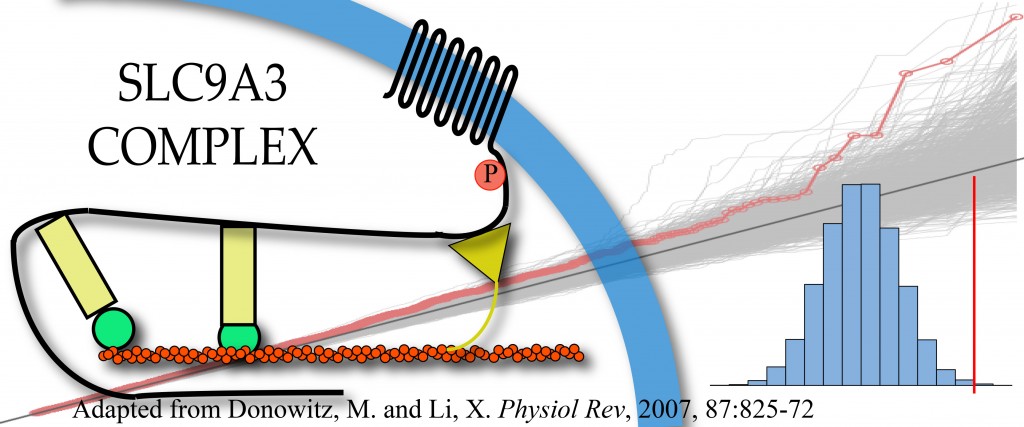
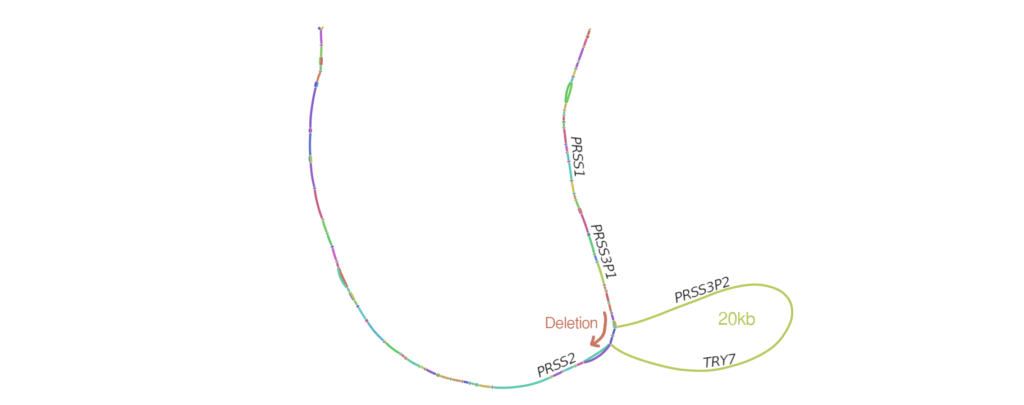
Cystic fibrosis affects multiple organs and is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Individuals with the same causal mutations can have very different disease severity across the affected organs, and these differences are explained by both environmental factors and other genes, referred to as modifier genes. We study the genomes of individuals with cystic fibrosis to try to identify which modifier genes contribute to disease severity, with the goal of identifying novel therapeutic targets and developing prognostic models to improve outcomes for all individuals living with cystic fibrosis.
Epilepsy & neurodevelopment
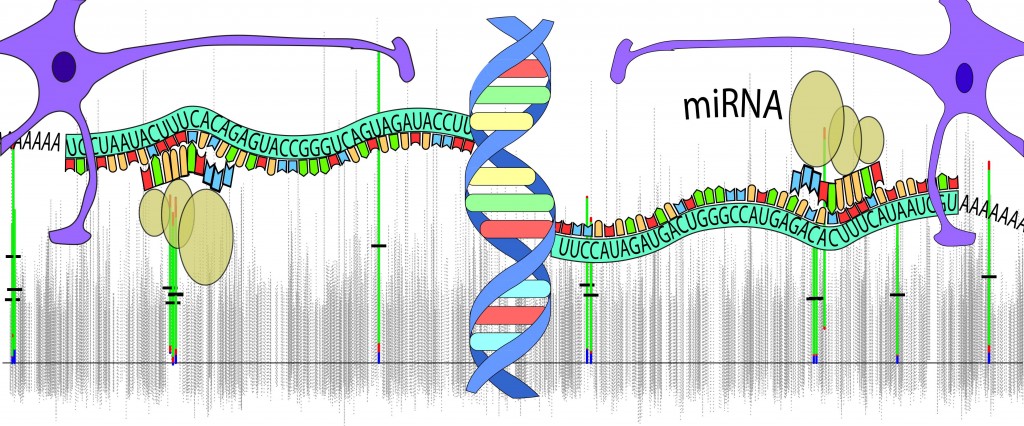
Epilepsy, defined as the tendency for recurrent unprovoked seizures, is a heterogeneous label encompassing both acquired and genetic aetiologies. Although the cause of many monogenic epilepsies is known, the challenge remains to find genetic contributions for common complex genetic epilepsies. Several studies have demonstrated that electroencephalograph (EEG) abnormalities and neuropsychological deficits typical in genetic generalised and focal epilepsies also occur in seizure-unaffected relatives. Using family and case-control population studies, we use these epilepsy-associated traits to identify genes that contribute to common, complex epilepsy syndromes.