Have a question?
Call us: 416-813-7654 ext. 202274
Email us: tammy.rayner@sickkids.ca, ruth.weiss@sickkids.ca
Internal Email: researchmri.team@sickkids.ca
SickKids Research MRI Core Facility
We support internal and external scientists and medical physicians’ research by providing access to dedicated, state-of-the-art Magnetic Resonance Imaging (MRI) equipment.
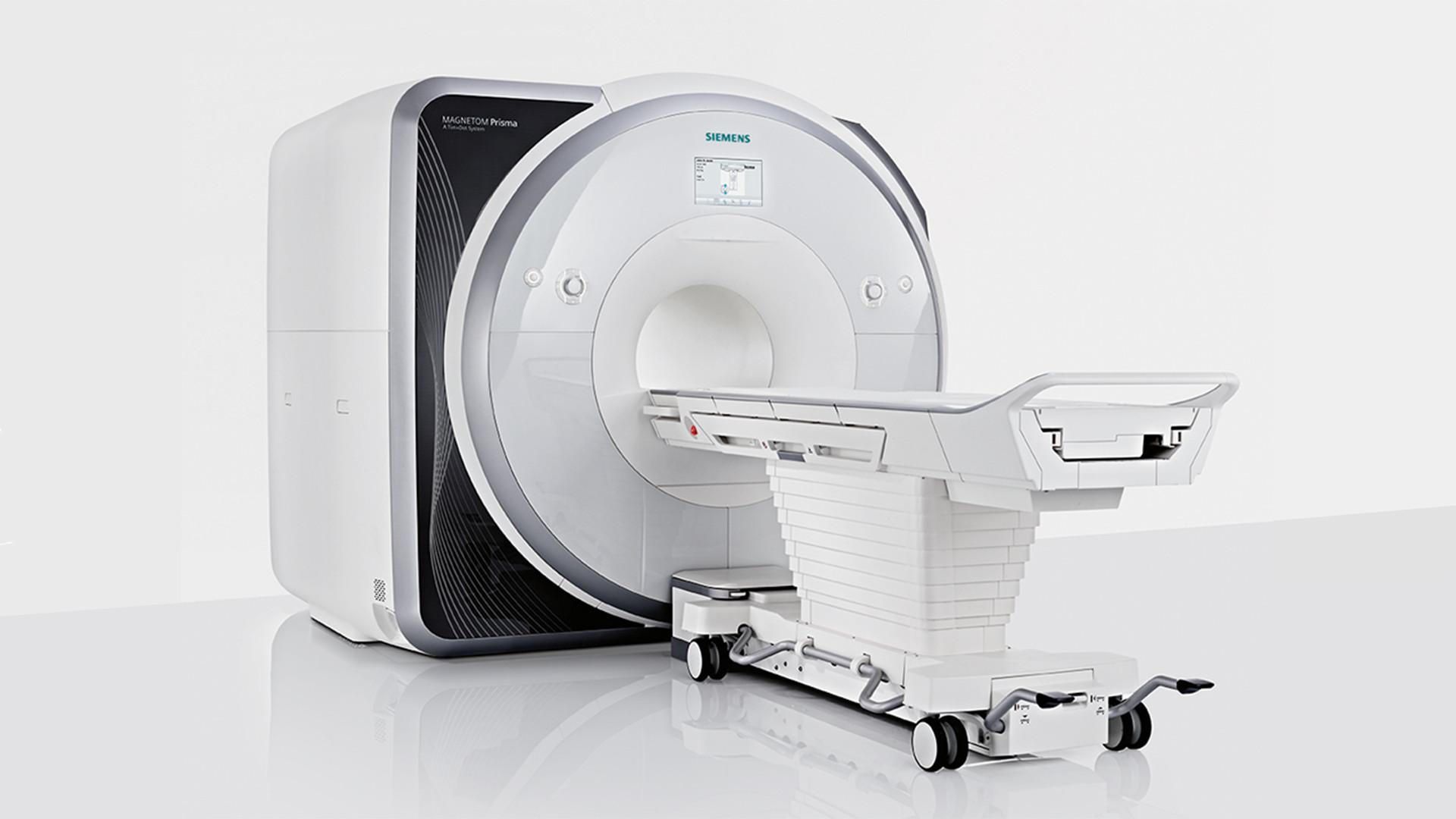
Led by a front-line team of child-friendly, experienced SickKids staff, we offer several key MR imaging services through our premier MRI system – the Siemens PrismaFit – to veteran and novice investigators interested in advancing their research.
Researchers worldwide use the facility for various types of work, employing the Siemens PrismaFit to scan wide varieties of age populations from fetal to geriatric, diagnosed populations, and more.
Our rates
For more information on facility costs, or to access our rate information in an alternative format, please contact us.
| MRI Research Grant | MRI Industry Sponsored | MRI Commercial Use | |
|---|---|---|---|
| Human | $250/30 min | ≥$600/30 min | N/A |
| Other | $150/30 min | To be determined | To be determined |
| MRT After Hours | $45/30 min | $90/30 min | $45/30 min |
| Gadovist | $75/7.5ml single use vial | $150/7.5ml single use vial | $75/7.5ml single use vial |
| Phantom Scan with MRT | $300/QA scan | $600/QA scan | $1000/hour (MRT Operated) |
| Phantom Scan no MRT | $200/QA scan | $400/QA scan | $500/hour (MRT Supervised) |
MRI Research Grant – Grant sponsored research studies are awards for conducting research that have undergone a competitive process and are peer reviewed. Funding is provided by but not limited to the government and charitable foundations.
MRI Industry Sponsored – Industry sponsored research studies are funded by a for-profit organization, performed by the non-profit Research MRI Core facility. The objective of the for-profit organization is to advance/develop a product and then distribute to the commercial market.
MRI Commercial Use – For profit companies who require use of MRI equipment for product and technical development.
Application process
- Before you can book the 3.0T Siemens PrismaFit, all research studies must obtain Research Ethics Board (REB) approval. Assistance from Legal services and/or the Research Institute Grants Management Office may be required for external research projects.
- Complete the Tri-Council Policy Statement (TCPS) Training, which is mandatory for all involved in human research.
- Applications are reviewed by a Research MRI technologist. You and/or your collaborators may be contacted for further protocol/sequence development and equipment testing if necessary.
- The Research MRI Application Form (PDF) can be completed and submitted entirely online. You’ll also need to fill out the Human Intake Form. A link to the Human Intake Form is also accessible in the MRI Application Form PDF.
- Once approved, you’ll receive access to our online self-scheduling system, Calpendo, for MRI and research assessment room bookings. Calpendo is only accessible from within the SickKids network (via VPN). Non-SickKids staff can contact the Research MRI team to set up their bookings.
Facility procedures
Tammy Rayner, MRT (MR)(R)
Senior Research MRI Technologist and Site Manager
Tammy has dedicated her career to SickKids MRI since 1999. She helped pioneer the research MRI facility in 2004, bringing her enthusiastic, creative, caring nature to volunteers and participants.
Ruth Weiss, MRT (MR)(R)
Senior Research MRI Technologist and Site Manager
Ruth has been practicing MRI since 2004 and joined the Research MRI team in 2007. Her knowledge and experience from other diagnostic disciplines provides additional compassion and sensitivity to the team and participants.
Rushanaa Thuraisingam
Patient Information Clerk
Rushanaa joined the team in 2024 with studies in psychology and kinesiology. With her education, fun and outgoing spirit, she brings a welcoming warmth to the families at the facility.
Brandon Zanette, PhD
Technical Innovation Investigator
Translational Medicine, SickKids Research Institute
The Hospital for Sick Children
Peter Gilgan Centre for Research and Learning
Christopher Macgowan, PhD
Scientific Director, Research MRI Core Facility
Senior Scientist, Translational Medicine, SickKids Research Institute
University of Toronto, Associate Professor, Department of Medical Biophysics
Kimberly Waite
Administrative Assistant to Dr. Chris Macgowan
Translational Medicine
The Hospital for Sick Children
Peter Gilgan Centre for Research and Learning
Contact us
For additional questions about the MRI facility including bookings, rates, equipment, services or availability, please get in touch!
416-813-7654 ext. 202274
The Hospital for Sick Children (SickKids)
Room S720
555 University Avenue
Toronto, ON
M5G 1X8
Contact us
For additional questions about the MRI facility including bookings, rates, equipment, services or availability, please get in touch!
416-813-7654 ext. 202274
The Hospital for Sick Children (SickKids)
Room S720
555 University Avenue
Toronto, ON
M5G 1X8