Will taking metformin improve thinking skills, especially memory and processing speed, in children with medulloblastoma?
What is being studied?
We are doing this study because we want to find out if giving metformin is better or the same as the standard of care given after treatment for medulloblastoma is finished. We are interested in seeing if metformin can:
- Improve your cognitive (thinking) skills (such as memory)
- Stimulate new growth to repair damaged brain tissue (white matter)
What will my child need to do?

Your child will be randomly assigned (50/50 chance) to one of two groups: either metformin or placebo. A placebo is a tablet with no medicine in it. Depending on which group your child is assigned to, they will either take metformin or placebo tablets one or two times a day for 16 weeks. Neither you nor the study team will know if they are taking metformin or placebo. This is known as double-blind.
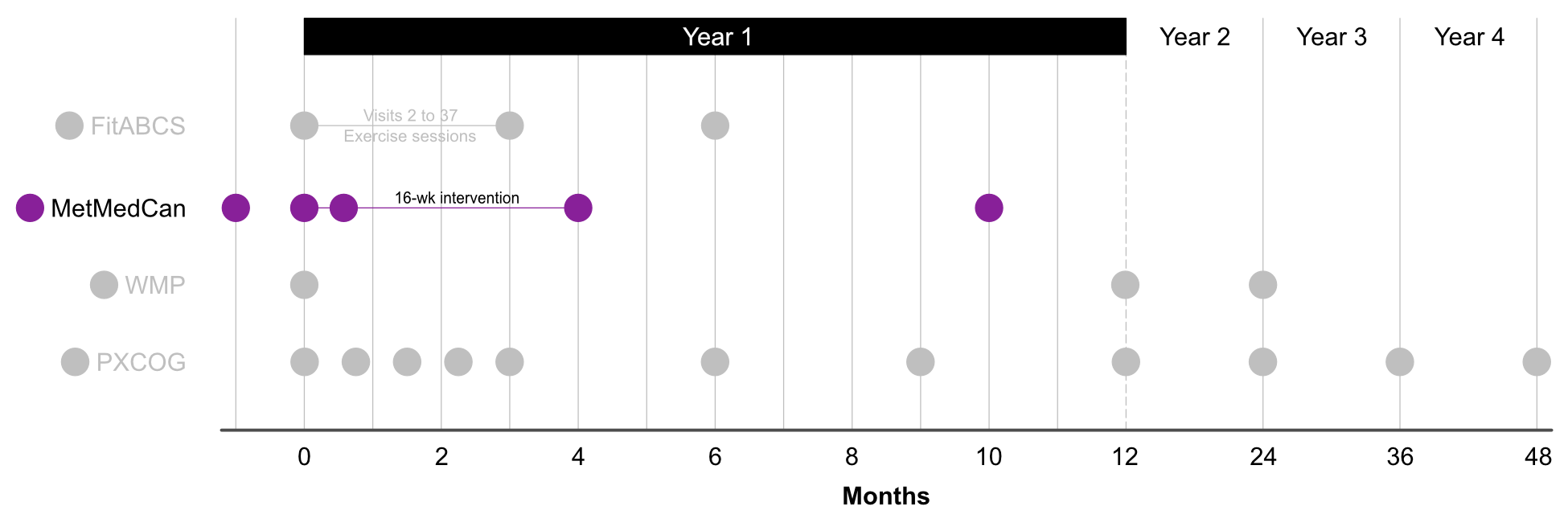
During the study, you and your child will be asked to come to SickKids for 5 visits across 40 weeks. These visits may include:
Who can participate in this study?
Your child can take part in the study if they:
- Have finished primary therapy for medulloblastoma (min. 3 weeks after end of therapy)
- Are between 7 and 17 years of age
- Have English as their native language or has had at least two years of schooling in English
- Are able to swallow tablets either whole, crushed, or via feeding tube
- Meet criteria for adequate organ function
Your child cannot take part in the study if they:
- Are unable to do an MRI without sedation
- Have an IQ <60 on screening test*
- Have a known hypersensitivity to metformin
- Have unstable and/or insulin-dependent (Type 1) diabetes
- Have a history of renal (kidney) disease or malfunction or congestive heart failure
- Are taking part in another cognitive rehabilitation intervention study
- Are currently using metformin
Reimbursement
- Reasonable out of pocket expenses, which may include transportation costs (e.g., mileage, public transportation, parking) and lunch on study visit days
- $15 gift card after study visit(s)
- Volunteer certificate (if applicable)
- Summary of cognitive (thinking) test results
- Newsletter with study updates twice a year
Fill in the form below for more information about the study.
Study timeline
Thank you for reading!
If you’re interested in participating or have any questions, please email/call with the address/number above or use the form below.