Structure-Based Immunogen Design
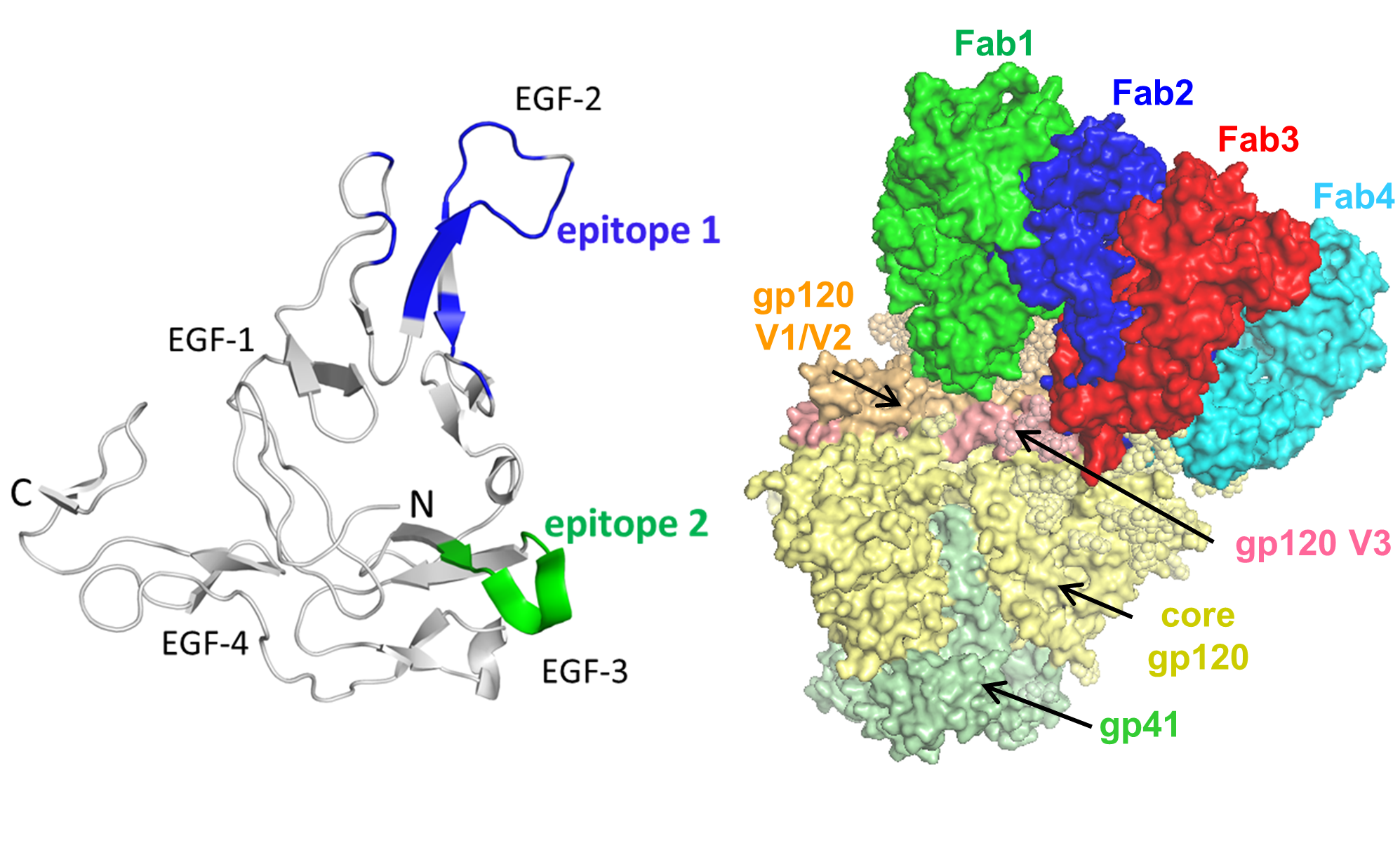
Malaria and HIV-1 are two of the most devastating and ongoing health burdens, particularly in the developing world. Protective vaccines against these pathogens remain elusive, in part because of our inability to induce by vaccination high-neutralizing antibody titers against conserved antigenic sites.
We use epitope-selective, structure-based protein design strategies to generate novel immunogens that elicit antibodies blocking the function of critical malaria and HIV-1 proteins.
Cell-Targeting Nanocages
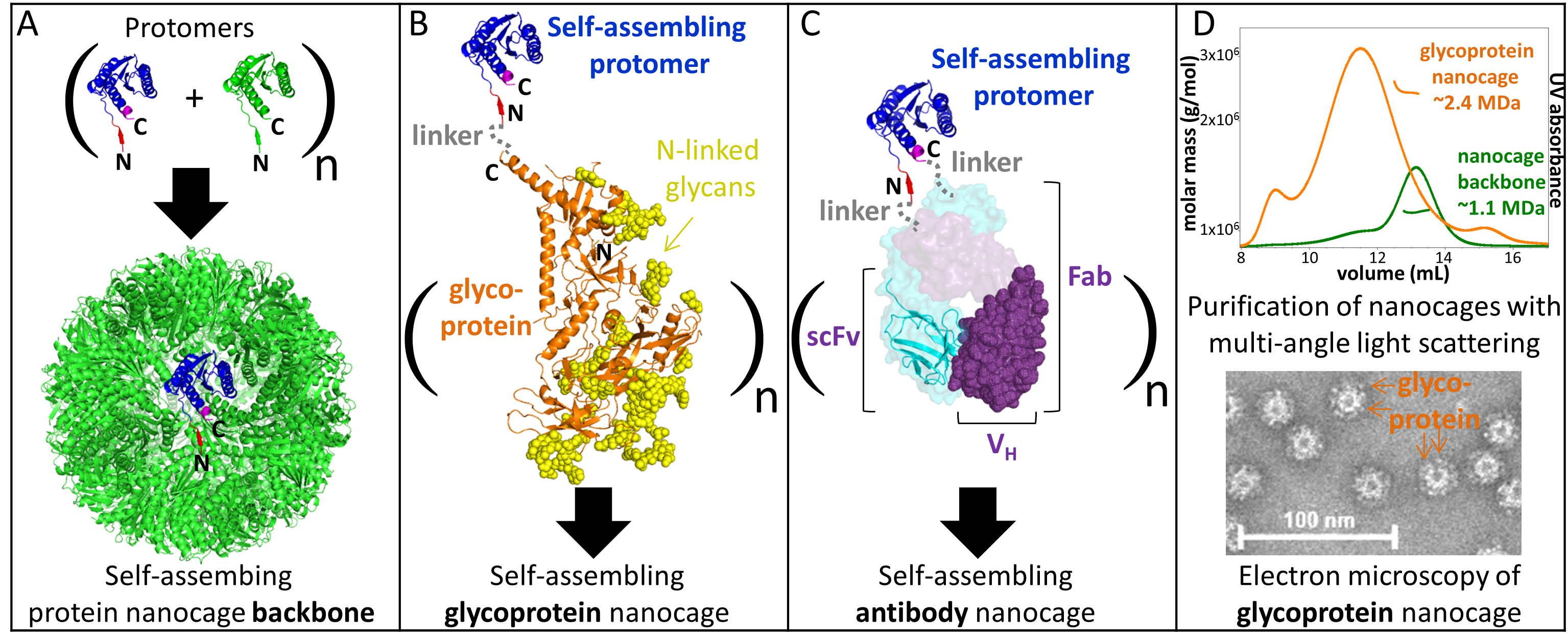
We study the fundamentals of protein nanoparticle assembly and use protein engineering and biophysical techniques to broaden the development of stable glycoprotein nanocages.
Our objective is to uncover the fundamentals of protein nanoparticle self-assembly to engineer the delivery of specific signals to target cells.
Structural Delineation of B cell Receptors
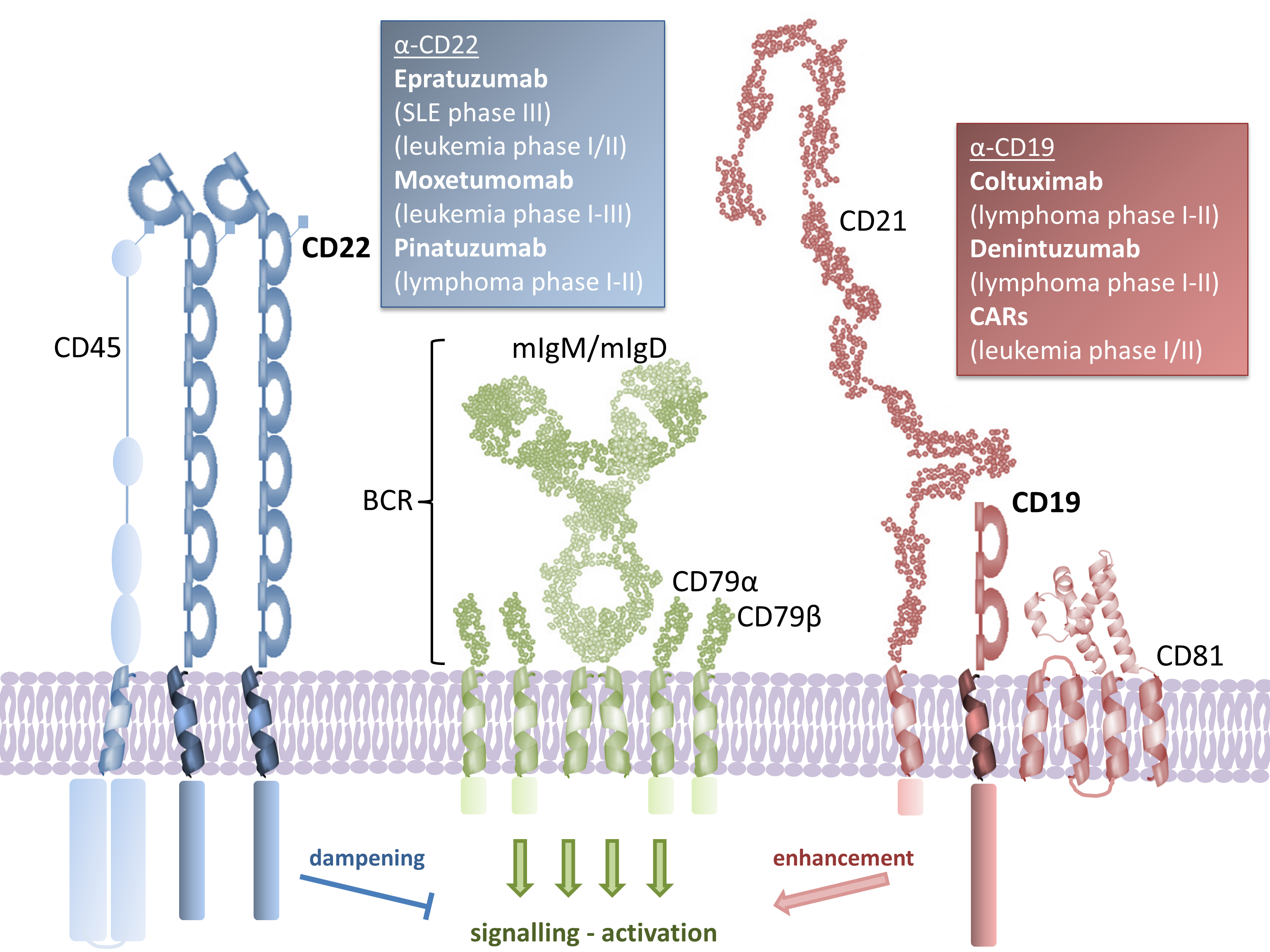
B cells are central to immunity. They protect hosts against pathogens primarily through the production of neutralizing antibodies. As such, surface receptors on B cells are prominent targets for the design of vaccines that can elicit high titers of neutralizing antibodies.
Conversely, dysregulation of B cell homeostasis can lead to autoimmune diseases, as well as devastating blood cancers, such as leukemias and lymphomas. B cell surface receptors are therefore also targets for the development of therapeutics that can deplete dysregulated B cells.
We use integrative structural biology techniques including X-ray crystallography and cryoEM to provide molecular details into the function and targeting of B cell receptors.
Platforms for Studies of Molecular Complexes
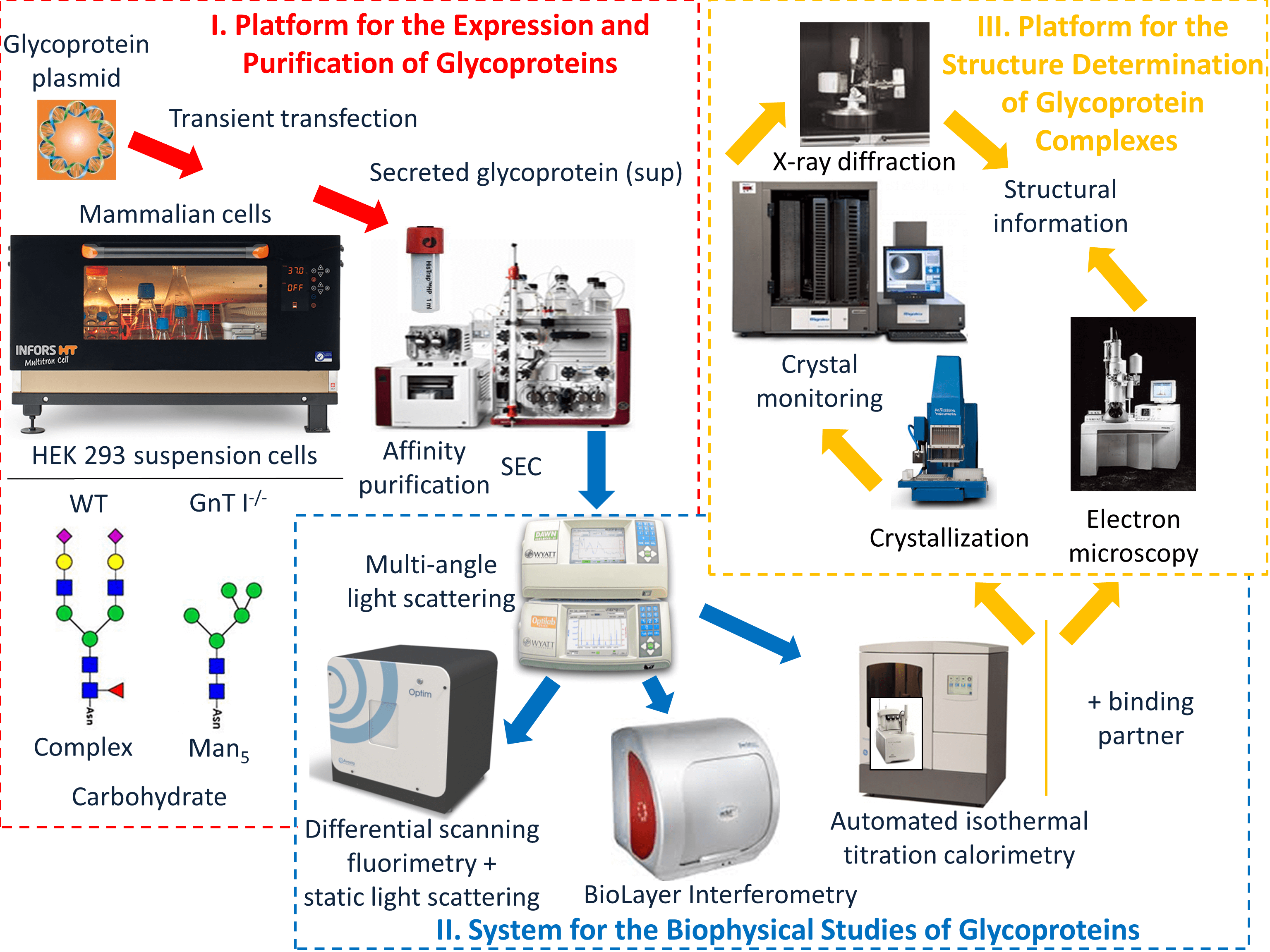
We have assembled high-throughput platforms in glycoprotein biochemistry and structural biology to study antibodies and glycoproteins in health and disease.
These include high-capacity incubator/shakers and high-performance purification equipment for the isolation of homogeneous glycoproteins from expression in mammalian/insect cells; high-throughput and micro-volume systems for the biophysical characterization of glycoproteins and their interactions with antibodies (Structural & Biophysical Core Facility); an automated crystallography infrastructure (Structural & Biophysical Core Facility) and state-of-the-art cryoEM equipment (Nanoscale Biomedical Imaging Facility) that includes a Titan Krios microscope equipped with a Falcon 4 direct electron detector.