Research in the Hayes lab
Our research incorporates developmental and cancer signaling, zebrafish modeling, human tissue culture, and xenograft transplantation approaches. We aim to integrate complementary model systems to identify and optimize targeted therapeutic approaches for paediatric cancers.
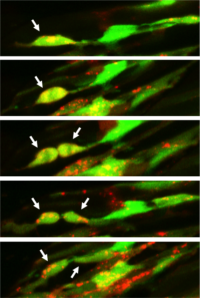
Confocal images of a rhabdomyosarcoma cell division in live zebrafish
Rhabdomyosarcoma (RMS) is a rare type of cancer that forms in soft tissue – specifically skeletal muscle and organs such as the bladder or uterus. RMS can occur at any age, but most often affects children. 60-70% of newly diagnosed patients with nonmetastatic disease can be cured using a combination of surgery, chemotherapy, and radiation. However, less than 20% of patients with metastatic disease are cured and due to a limited understanding of what drives RMS progression, very few targeted therapies currently exist.
RMS can be effectively modeled in zebrafish to understand the role for specific genetic signaling pathways in vivo (Langenau et al. 2007, Hayes et al. 2018, Hayes and Ignatius et al. 2018). Using fluorescent transgenes, tumor cells plus the surrounding tumour microenvironment can be directly visualized to understand how RMS spreads throughout the body. Tumor growth and metastasis can be quantified in real-time, with candidate molecular mechanisms assessed using transgenic gene over-expression, CRISPR/Cas9-mediated mutagenesis, and/or drug inhibition. Our lab uses these approaches and human cells to understand what makes certain RMS tumours more aggressive and how drugs can be used to inhibit progression.
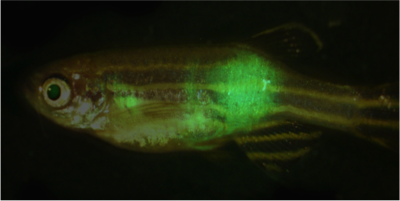
Adult zebrafish with GFP-labeled metastatic rhabdomyosarcoma

Immune-deficient zebrafish engrafted with human rhabdomyosarcoma (RD) cells
Neuroblastoma (NB) is the most common extra-cranial solid tumour in children, with patients with high-risk disease experiencing long-term survival rates of less than 40% due to metastases that frequently recur. While recent progress has been made to define biological heterogeneity using high-throughput sequencing and different modeling approaches, the roles for specific genetic mechanisms in tumour initiation, growth and metastasis remain poorly understood. Amplification of the MYCN oncogene (MYCNA) is detected in approximately 20% of NB (40% of high risk cases) and is a major predictor of poor clinical outcome. However, MYCNA tumours display diverse clinical responses ranging from complete resolution to fatal metastatic disease.
In collaboration with Drs. Meredith Irwin and David Kaplan, we aim to uncover mechanisms associated with poor prognosis for MYCNA NB. Our group utilizes an established zebrafish model of NB (Tg(dbh:EGFP-MYCN), Zhu et al. 2012) to test the role for candidate patient mutations and their contribution to NB progression. Furthermore, we are able to orally gavage zebrafish with clinically relevant drug combinations to test efficacy for patients with high-risk NB.

Zebrafish with GFP-labeled neuroblastoma
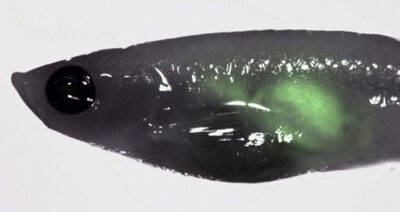
Immune-deficient zebrafish engrafted with human NB (Kelly) cells
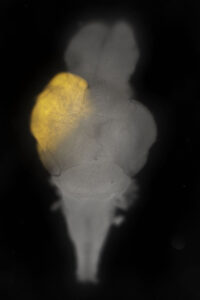
Whole zebrafish brain at 30 days post fertilization with an mScarlet-labeled tumour
In collaboration with Dr. Bret Pearson, we built a zebrafish brain tumor model that recapitulates aspects of human glioblastoma. Up to 25% of our animals develop tumors prior to 30 days or life, which means that we can rapidly assess candidate genes and/or drugs in vivo for their effects on tumor initiation, growth and/or relapse. In the future, we aim to assess roles for the tumor microenvironment during tumor initiation and screen candidate chemical compounds for inhibitors of tumor growth that may effectively treat patients with otherwise fatal disease.
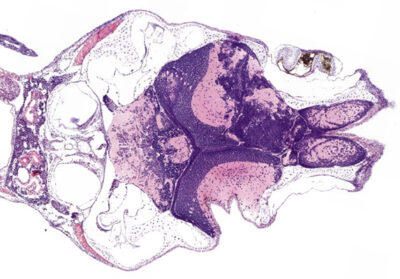
Hematoxylin and eosin (H&E) staining of a tumour-positive zebrafish brain
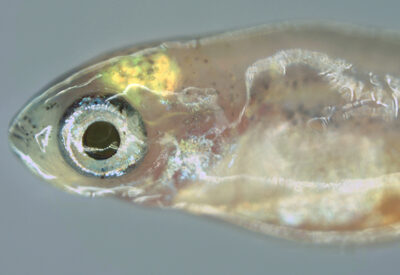
Zebrafish at 30 days post fertilization with a fluorescently-labeled brain tumour

