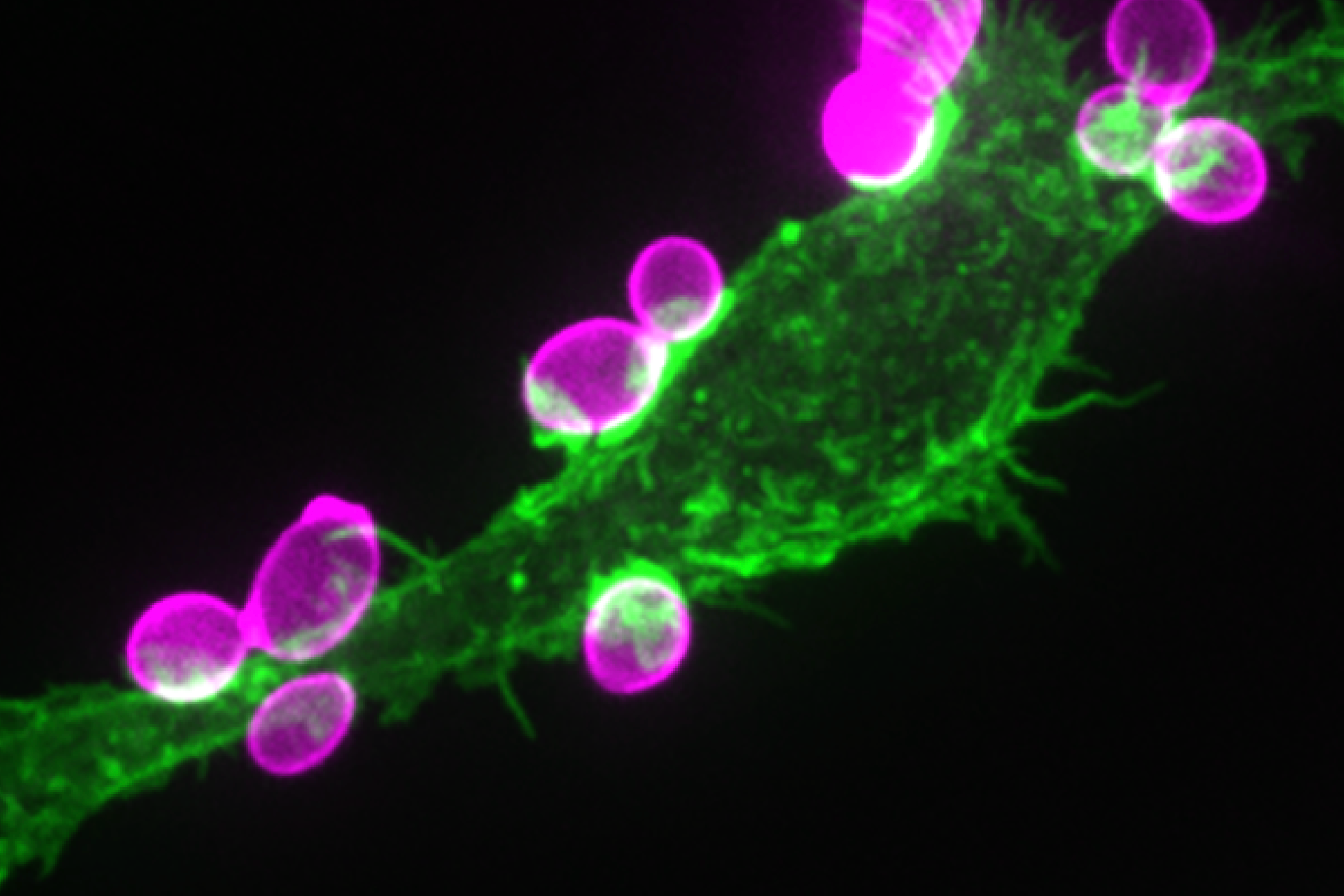
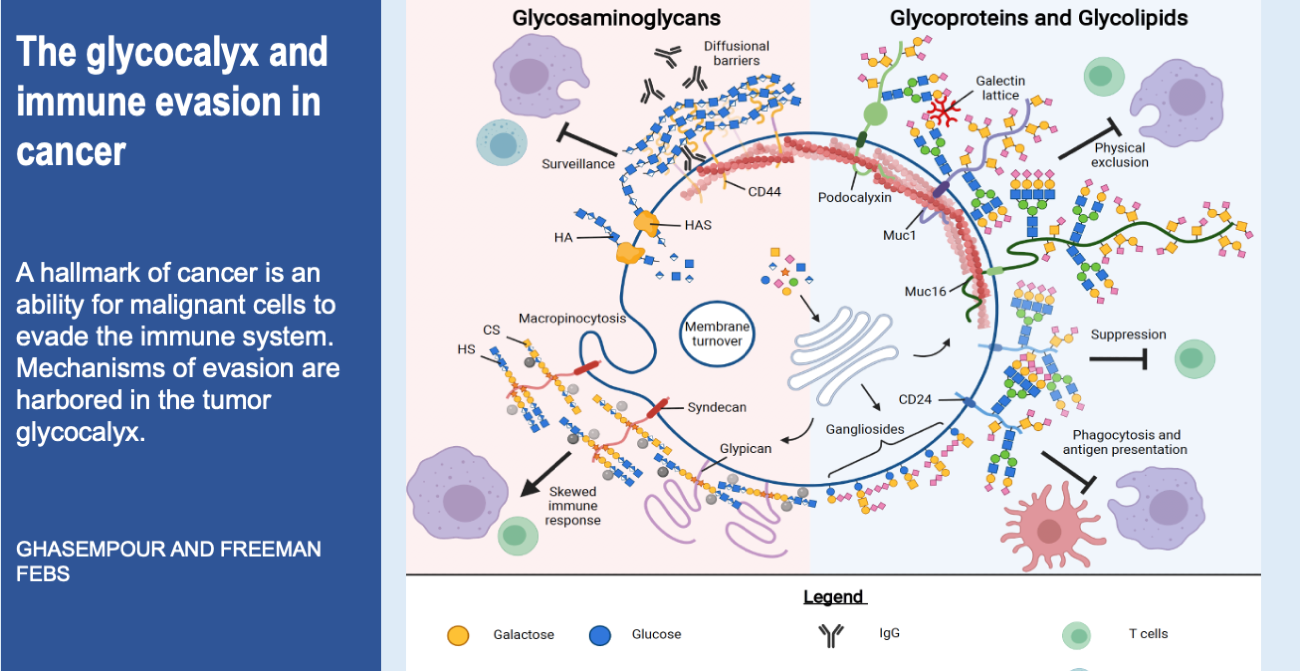
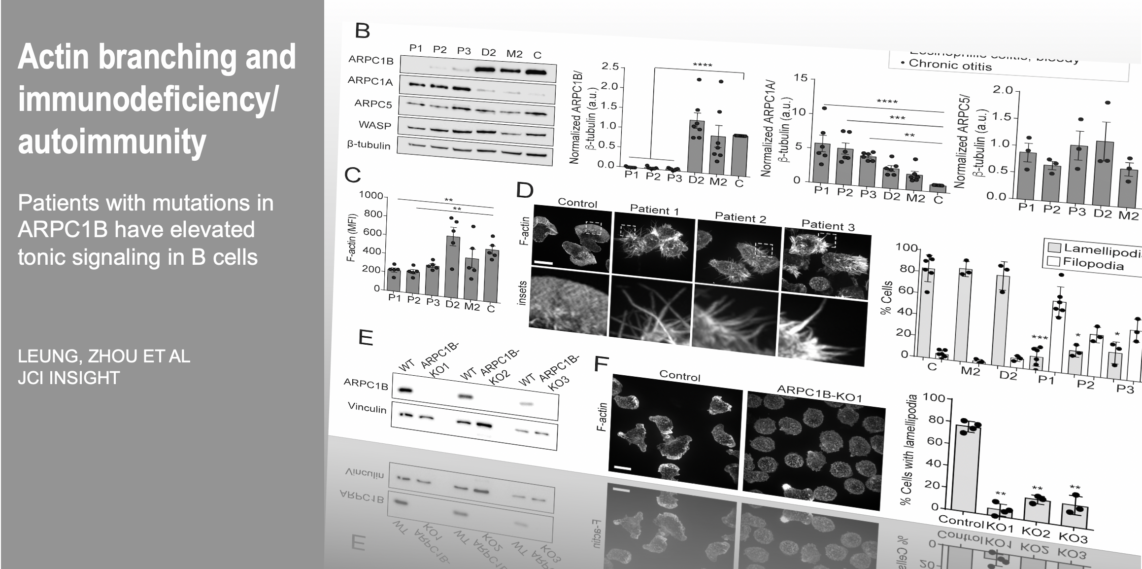
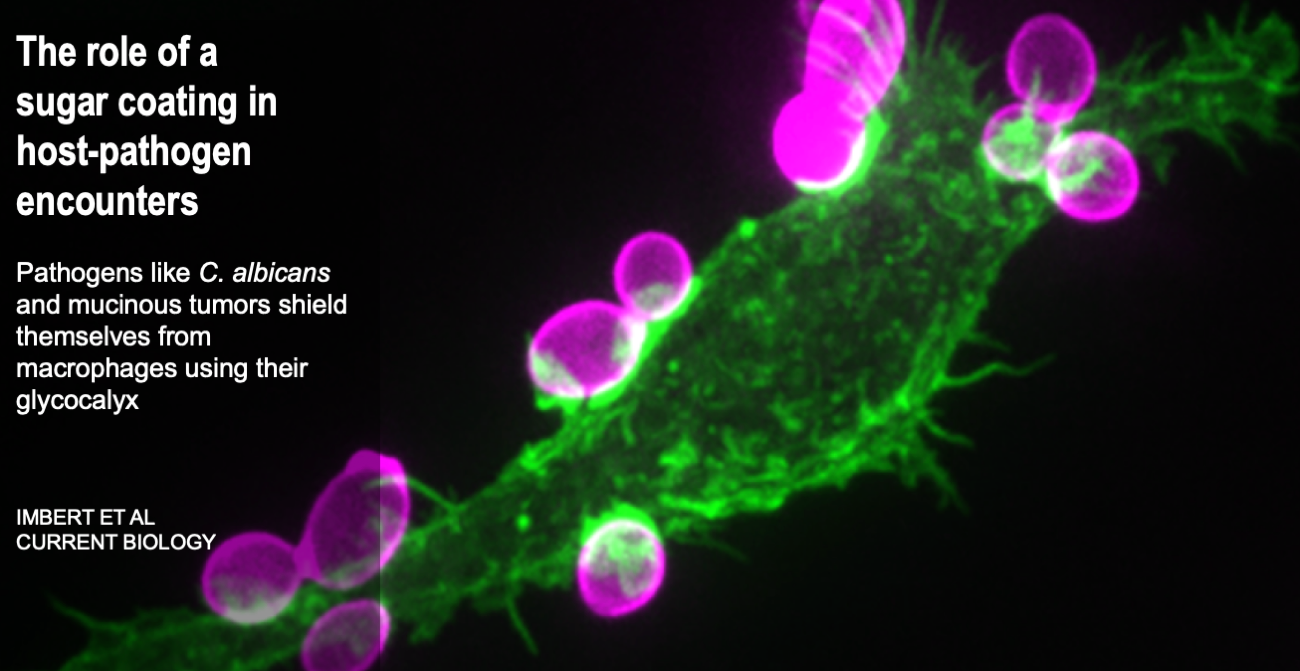
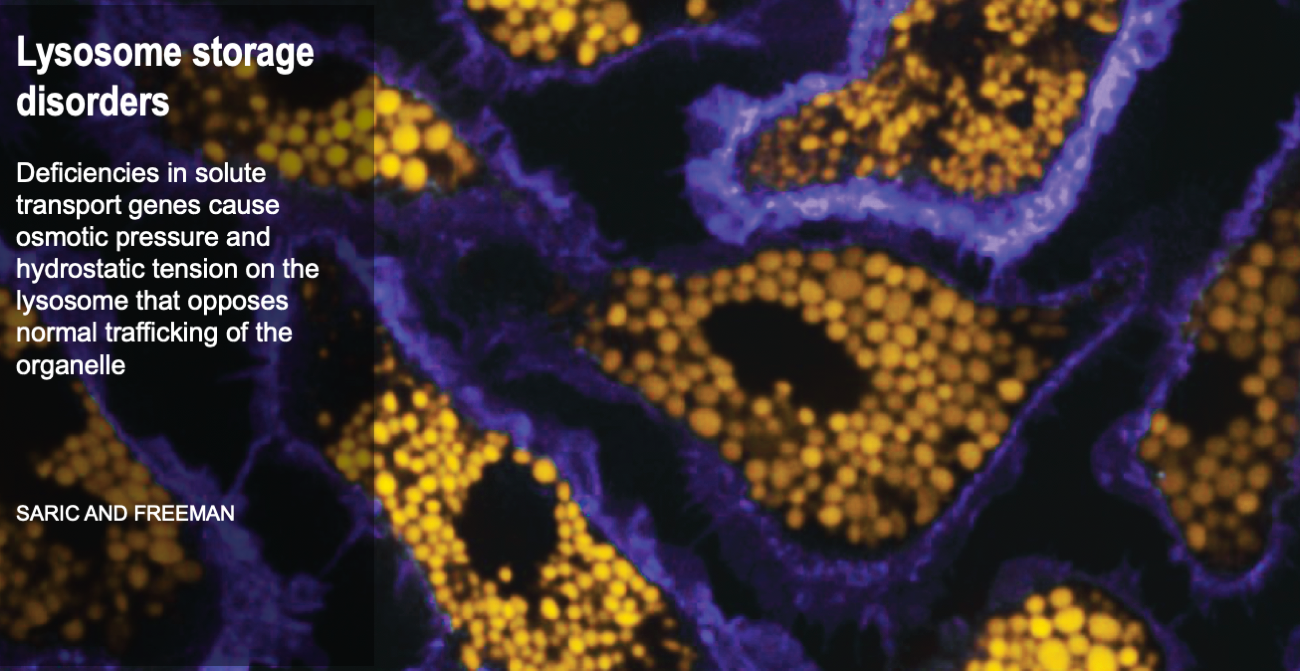
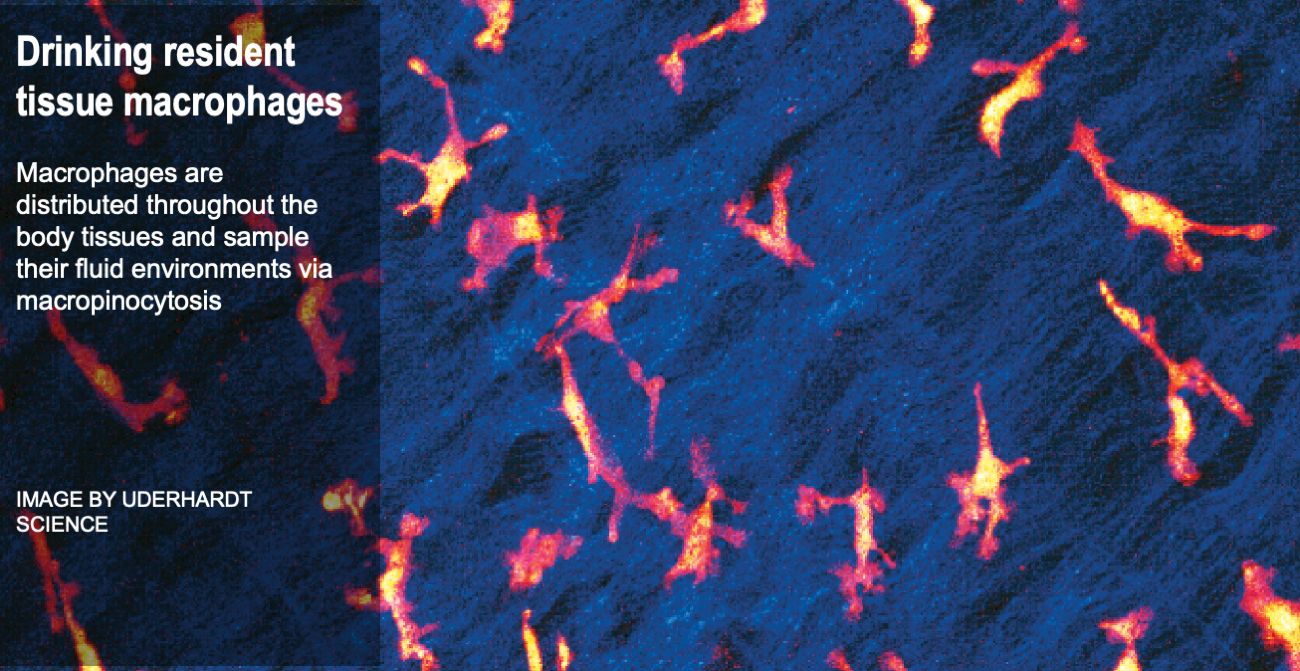
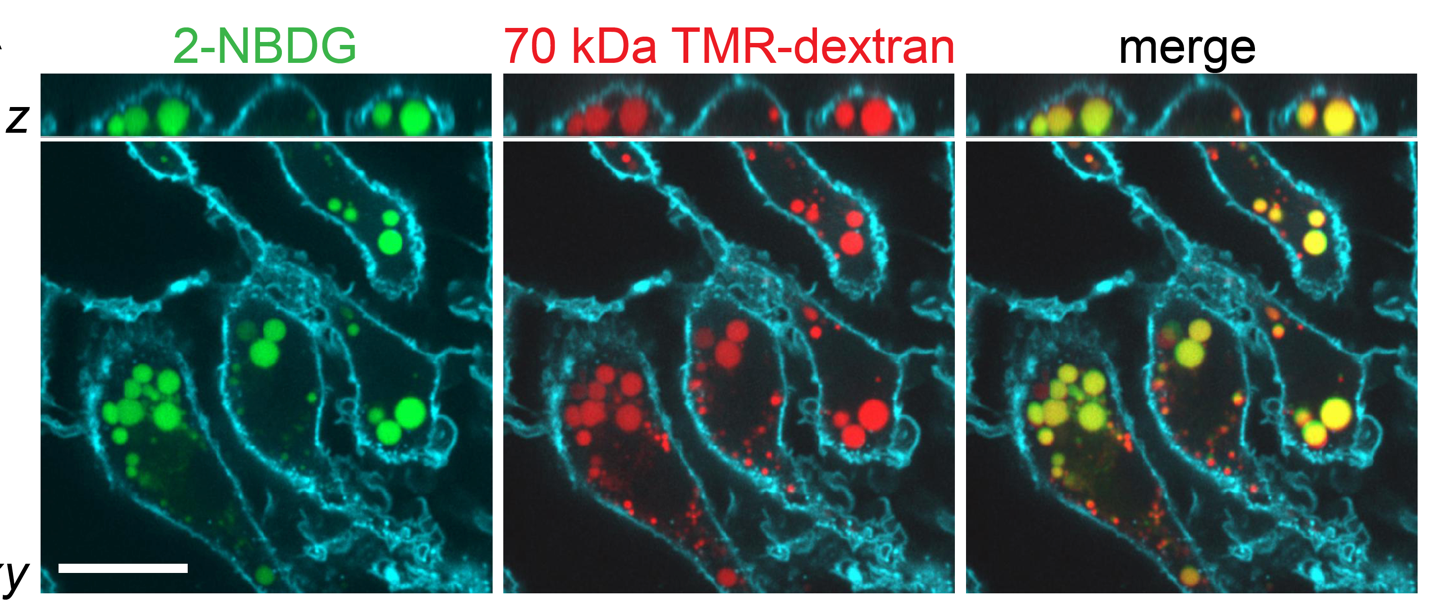
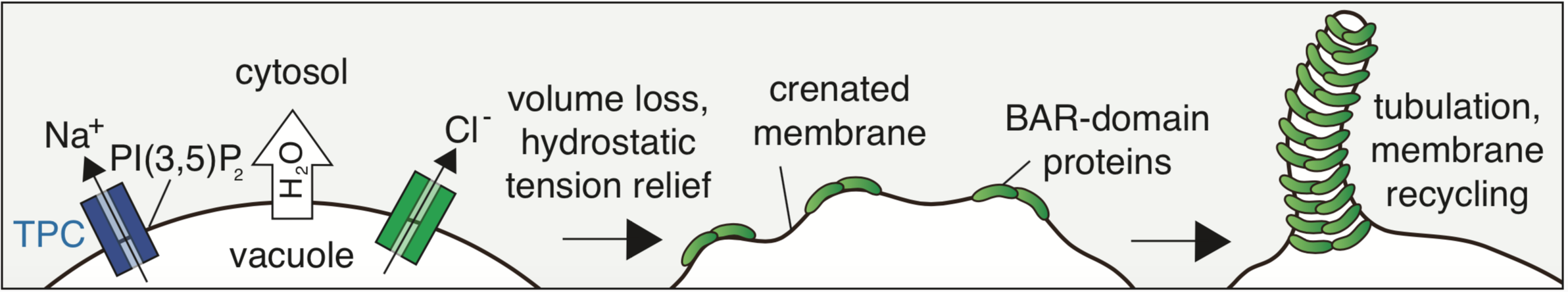
Cells are the smallest independent units of plants and animals. Virtually all human organs and tissues have specialized, sentinel cells (macrophages) that are dedicated to protecting the body from infection, damage, or toxins. These cells are part of the innate immune system.
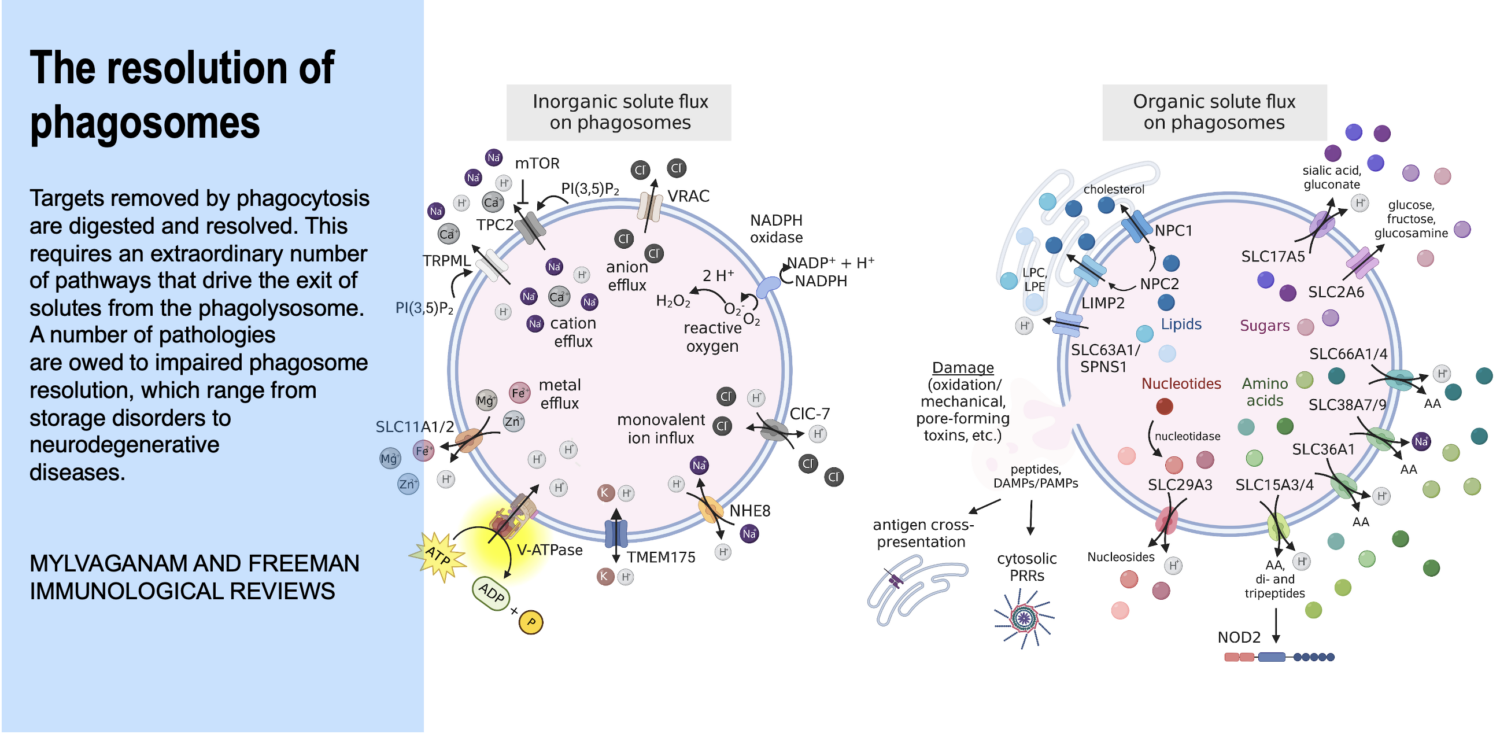
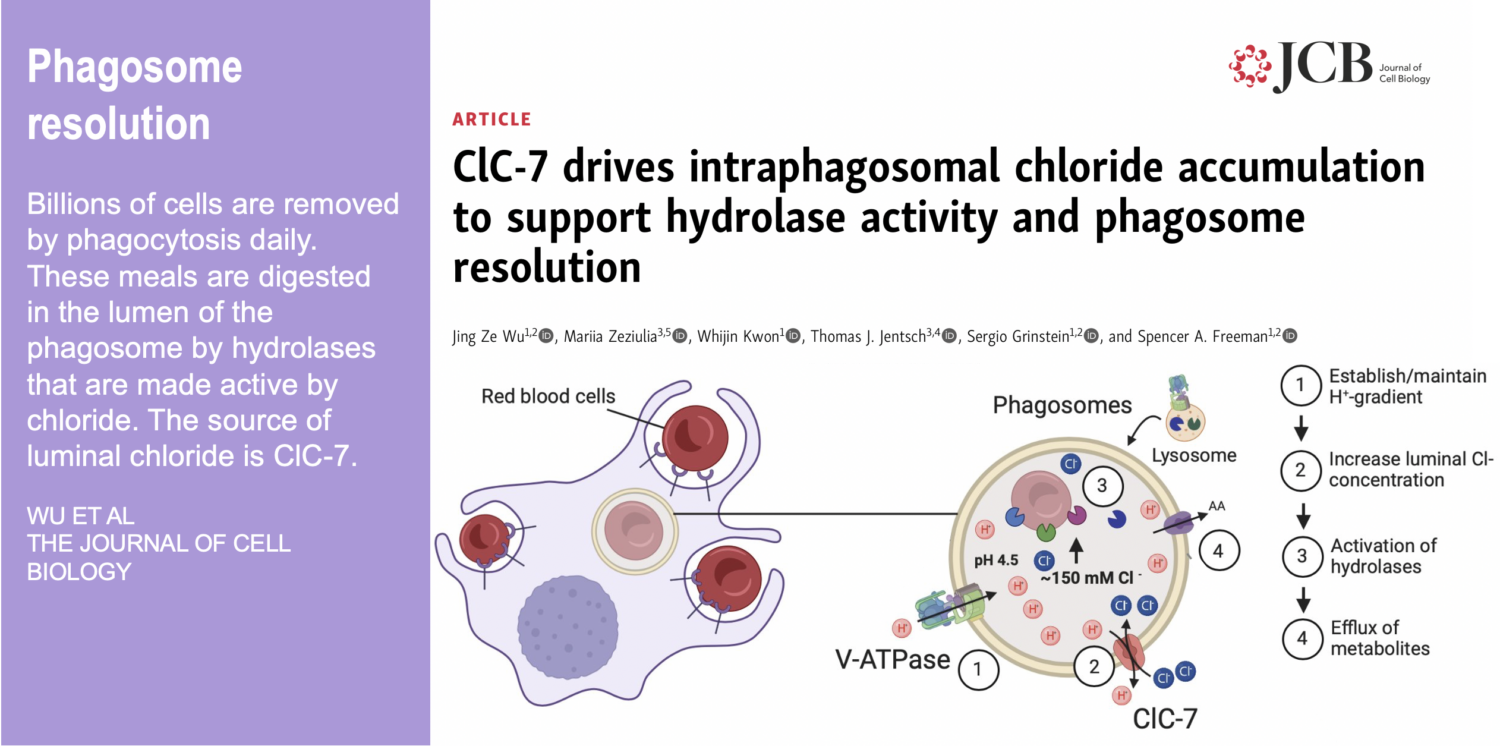
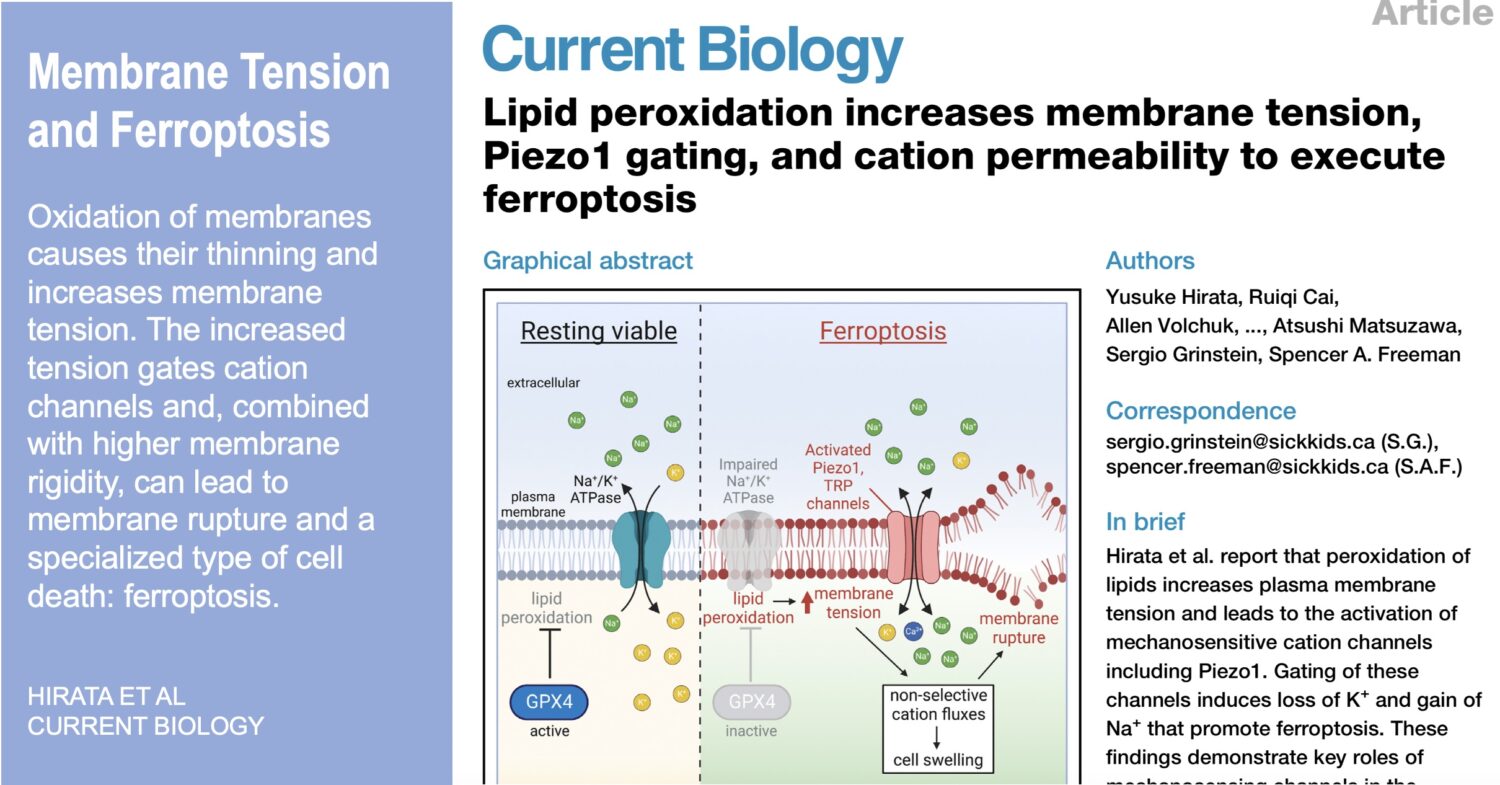
While the innate immune system protects us from infection, orchestrates wound healing, and eliminates cancer, even in the absence of obvious infection or injury, macrophages maintain homeostasis and support tissue metabolism. They do so by turning over and rejuvenating components of tissues by specialized types of endocytosis, preventing the accumulation of damage. This requires an enormous capacity for these cells to handle the internalized cargoes/solutes from their microenvironment. Defects in these pathways contribute to a wide-segment of congenital and acquired diseases including storage disorders, inflammation, and atherosclerosis.
We are working to understand cellular and subcellular mechanisms underlying the everyday surveillance functions of macrophages.